What are these nuggets? What to do?
 lkeigwin
Posts: 16,892 ✭✭✭✭✭
lkeigwin
Posts: 16,892 ✭✭✭✭✭
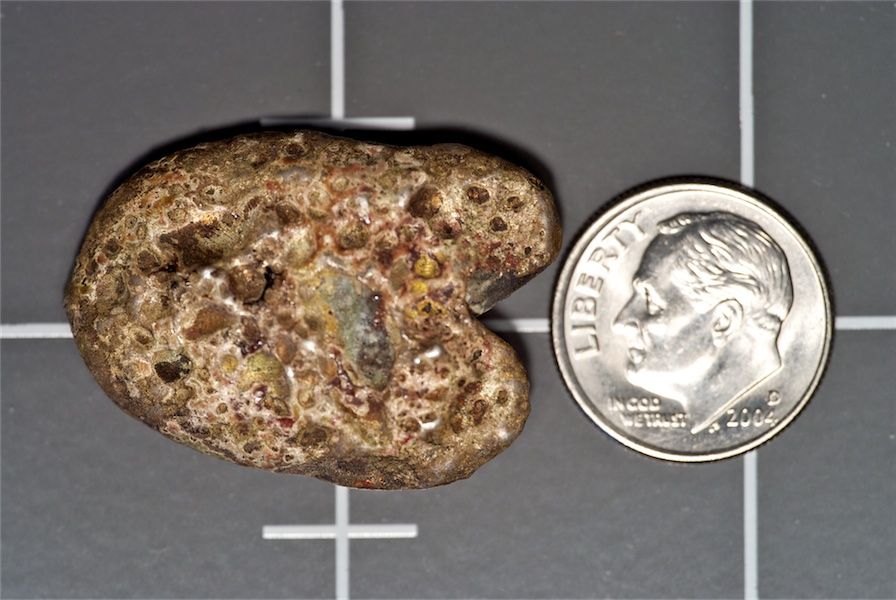
The photos below are of two nuggets(?) I acquired with a small collection of circulated coins. They belonged to a friend of a friend who lived in Alaska for many years. I don't know with certainty but I am guessing he found them there. (I also have a few wedges of what I believe is copper.)
The first one weighs 11.6g and is 1"x.9"x.17" thick. (In the 3rd photo each is resting on a chunk of copper.)
The second one weighs 25.6g and is 1.20"x.8"x.25" at its thickest.
Questions:
1) What do you think these are?
2) What is a practical way of finding out? What's it cost? Is it worth it?
3) In what form they are best sold?
I don't know much about precious metals other than the going rates.
Thanks for the help and advice.
Lance.





The first one weighs 11.6g and is 1"x.9"x.17" thick. (In the 3rd photo each is resting on a chunk of copper.)
The second one weighs 25.6g and is 1.20"x.8"x.25" at its thickest.
Questions:
1) What do you think these are?
2) What is a practical way of finding out? What's it cost? Is it worth it?
3) In what form they are best sold?
I don't know much about precious metals other than the going rates.
Thanks for the help and advice.
Lance.






Coin Photography Services / Everyman Registry set / BHNC #213
0
Comments
These nuggets look smooth from erosion, probably water. The sharp angular piece you see (3rd pic for each nugget) is a chunk of copper.
Lance.
<< <i>These do not look like nuggets to me, they look melted like from mining fines or scrap. >>
I'm not sure and that's why I'm asking. The top one looks like placer gold, such as these.
Lance.
Making Dore button
<< <i>
<< <i>These do not look like nuggets to me, they look melted like from mining fines or scrap. >>
I'm not sure and that's why I'm asking. The top one looks like placer gold, such as these.
Lance.
Respectfully, I disagree. The original pictures look nothing like your natural nuggets. The first has obvious shrinkage rings and the second still displays melted borax flux. They both certainly contain gold however and I'd love to refine the first for you. The second needs help as it contains lots of copper.
Many members on this forum that now it cannot fit in my signature. Please ask for entire list.
<< <i>These do not look like nuggets to me, they look melted like from mining fines or scrap. >>
Worry is the interest you pay on a debt you may not owe.
"Paper money eventually returns to its intrinsic value---zero."----Voltaire
"Everything you say should be true, but not everything true should be said."----Voltaire
<< <i>Respectfully, I disagree. The original pictures look nothing like your natural nuggets. The first has obvious shrinkage rings and the second still displays melted borax flux. They both certainly contain gold however and I'd love to refine the first for you. The second needs help as it contains lots of copper. >>
Good information. Thanks. I assume "shrinkage rings" occur when gold cools and solidifies? I don't know about this stuff so I appreciate the help.
Since they're not nuggets then there's no collectible value, correct? No harm in getting them acid-tested? Is that the right thing to do?
I'm really not interested in collecting gold this way. I'm just looking to sell it and move on but I'm trying to figure out what they're worth.
Lance.
would melt them into a button on site. Another word for fines is dust. Certainly don't want to
throw them out and easy to get lost or whatever. So, just fire up a roaring campfire and
melt those fines into a button.
My wag.
bob
It's hard to be 100% certain since color is likely off because of camera and monitors. However, none appear to be gold. Based on color and flow lines etc. My guess is copper/brass alloys. Simple acid test would tell you though. A fire assay where I live only costs me $30 if you're still not sure if there is any gold content.
<< <i>Intentionally man made "nugget". When fines from placering are small and easily lost a miner
would melt them into a button on site. Another word for fines is dust. Certainly don't want to
throw them out and easy to get lost or whatever. So, just fire up a roaring campfire and
melt those fines into a button. >>
Interesting theory but I doubt that a camp fire can get hot enough to melt gold which has a very high melting point at 1947 degrees Fahrenheit.
Worry is the interest you pay on a debt you may not owe.
"Paper money eventually returns to its intrinsic value---zero."----Voltaire
"Everything you say should be true, but not everything true should be said."----Voltaire
<< <i>
<< <i>Intentionally man made "nugget". When fines from placering are small and easily lost a miner
would melt them into a button on site. Another word for fines is dust. Certainly don't want to
throw them out and easy to get lost or whatever. So, just fire up a roaring campfire and
melt those fines into a button. >>
Interesting theory but I doubt that a camp fire can get hot enough to melt gold which has a very high melting point at 1947 degrees Fahrenheit. >>
I see that you have never attempted this. It is quite easily done over an open campfire. Well, not the open campfire that
you experience in the woods, perhaps. But, none the less, it is not hard to get the temp high enough for a short few seconds
to make buttons from fines. A good chimney, wide at the base and narrow at the top and a forced air source (blower or
bellows (mechanical or hand)) will do the job just fine. A well ventilated controlled fire can get well above 2000 degrees F.
Plenty of heat for melting gold, silver and if you work at it Platinum.
bob
<< <i>
<< <i>
<< <i>Intentionally man made "nugget". When fines from placering are small and easily lost a miner
would melt them into a button on site. Another word for fines is dust. Certainly don't want to
throw them out and easy to get lost or whatever. So, just fire up a roaring campfire and
melt those fines into a button. >>
Interesting theory but I doubt that a camp fire can get hot enough to melt gold which has a very high melting point at 1947 degrees Fahrenheit. >>
I see that you have never attempted this. It is quite easily done over an open campfire. Well, not the open campfire that
you experience in the woods, perhaps. But, none the less, it is not hard to get the temp high enough for a short few seconds
to make buttons from fines. A good chimney, wide at the base and narrow at the top and a forced air source (blower or
bellows (mechanical or hand)) will do the job just fine. A well ventilated controlled fire can get well above 2000 degrees F.
Plenty of heat for melting gold, silver and if you work at it Platinum.
bob >>
You are describing a forge which would do the job but a camp fire doesn't include the forced air and chimney.
Worry is the interest you pay on a debt you may not owe.
"Paper money eventually returns to its intrinsic value---zero."----Voltaire
"Everything you say should be true, but not everything true should be said."----Voltaire
<< <i>It's hard to be 100% certain since color is likely off because of camera and monitors. However, none appear to be gold. Based on color and flow lines etc. My guess is copper/brass alloys. Simple acid test would tell you though. A fire assay where I live only costs me $30 if you're still not sure if there is any gold content. >>
It is sometimes said that one should be willing to back one's opinion with one's own cash. In this instance I disagree sufficiently to offer to do so.I'd pay 83% of the gross weight of faux-nugget #1 as if it were pure gold. The second I'm not sure but it does (IMO) contain gold.
<< <i>
<< <i>
<< <i>
<< <i>Intentionally man made "nugget". When fines from placering are small and easily lost a miner
would melt them into a button on site. Another word for fines is dust. Certainly don't want to
throw them out and easy to get lost or whatever. So, just fire up a roaring campfire and
melt those fines into a button. >>
Interesting theory but I doubt that a camp fire can get hot enough to melt gold which has a very high melting point at 1947 degrees Fahrenheit. >>
I see that you have never attempted this. It is quite easily done over an open campfire. Well, not the open campfire that
you experience in the woods, perhaps. But, none the less, it is not hard to get the temp high enough for a short few seconds
to make buttons from fines. A good chimney, wide at the base and narrow at the top and a forced air source (blower or
bellows (mechanical or hand)) will do the job just fine. A well ventilated controlled fire can get well above 2000 degrees F.
Plenty of heat for melting gold, silver and if you work at it Platinum.
bob >>
You are describing a forge which would do the job but a camp fire doesn't include the forced air and chimney. >>
Perry is exactly right.....a CAMPFIRE will not melt gold fines.
<< <i>
<< <i>It's hard to be 100% certain since color is likely off because of camera and monitors. However, none appear to be gold. Based on color and flow lines etc. My guess is copper/brass alloys. Simple acid test would tell you though. A fire assay where I live only costs me $30 if you're still not sure if there is any gold content. >>
It is sometimes said that one should be willing to back one's opinion with one's own cash. In this instance I disagree sufficiently to offer to do so.I'd pay 83% of the gross weight of faux-nugget #1 as if it were pure gold. The second I'm not sure but it does (IMO) contain gold. >>
WOW!!!!!!!!
Will you extend such a generous offer to me if I post pictures of items that are golden in color too? I could be a millionaire in about 15 mins if you agree!
<< <i>
<< <i>
<< <i>These do not look like nuggets to me, they look melted like from mining fines or scrap. >>
I'm not sure and that's why I'm asking. The top one looks like placer gold, such as these.
Lance.
Respectfully, I disagree. The original pictures look nothing like your natural nuggets. The first has obvious shrinkage rings and the second still displays melted borax flux. They both certainly contain gold however and I'd love to refine the first for you. The second needs help as it contains lots of copper. >>
The big number in the upper right look like a 'fabulous fake' gold plated copper. Fun pieces btw
<< <i>
<< <i>
<< <i>It's hard to be 100% certain since color is likely off because of camera and monitors. However, none appear to be gold. Based on color and flow lines etc. My guess is copper/brass alloys. Simple acid test would tell you though. A fire assay where I live only costs me $30 if you're still not sure if there is any gold content. >>
It is sometimes said that one should be willing to back one's opinion with one's own cash. In this instance I disagree sufficiently to offer to do so.I'd pay 83% of the gross weight of faux-nugget #1 as if it were pure gold. The second I'm not sure but it does (IMO) contain gold. >>
WOW!!!!!!!!
Will you extend such a generous offer to me if I post pictures of items that are golden in color too? I could be a millionaire in about 15 mins if you agree! >>
No I'm afraid that in your case I would not Phil. But I'd still love to see the pix.
-----
Kidding aside, this IMO has sufficient gold that I would make my offer good. Sometimes I'm wrong of course but that's why I'm considered unique on this forum........ I'm the one who makes mistakes! Really makes me standout from the background.
There clearly is at least 2 inclusions of copper, but the overall appearance ( again to me) is that of an attempt to melt together a number of smaller bits and whoever did it did a fairly decent job. Not perfect, one can see some white swirls on the 'obverse' too.
-----
The second was not so sucessfull an attempt. More differentiation of the metals especially as they cooled and around the edge.
------
If these are plated copper, how is it that one the size of a dime and three times as thick weighs 25.6 grams? Maybe it's plated uranium.
------
Considering the story, what I see in the pix, and my own uncommonly good judgement , I say it's worth $990.55 ( just the first one). Send it off to Midwest and see what they'll pay you.
John, your offer is generous and I almost took you up on it. When you cited the weight I looked back at my OP and noticed order of the photos is backwards. Damn! Sorry. As you can see, the second piece is bigger and thicker. So the piece you were interested in is 11.6g. I'm sure your feeling is different now.
I'll fix the OP not by changing the order of pix since a lot of folks referred to them by this order, and instead change the text.
Based on opinions here I'm skeptical about gold.
Lance.
<< <i>
<< <i>These do not look like nuggets to me, they look melted like from mining fines or scrap. >>
I'm not sure and that's why I'm asking. The top one looks like placer gold, such as these.
Lance.
Go to you tube and type in smelting gold, and there are plenty of videos that look like your pics. It seems they use wood to pour the melt into a lot of time and the result is very similar to yours.
Successful Trades: Swampboy,