Well... this is refreshing..... Some people actually doing real experiments.... Though I am not sure they have been done under controlled conditions, at least we may be stepping beyond the state of 'uninformed opinion'.
Rick Snow has posted in depth information on the 'blue' copper phenomenon..... those who missed it, should look it up. Certainly do some experimentation -valuable experience. I spent a couple of years doing experiments with silver tarnish. For those too busy to do their own tests, for copper, please read Rick's treatise on the subject. Cheers, RickO
That is in no way a circulation/business strike, but that color is natural and not caused by MS70. I know what blue from MS70 usage looks like. If someone can show me an actual business strike with blue color that doesn't look like what I know blue from MS70 to look like, I will amend my statements, but I'm yet to see one.
I think there are about eight different dies/die states for this very tricky date, and business strike were obviously struck from proof dies. Are @You an EAC guy?
"People sleep peaceably in their beds at night only because rough men stand ready to do violence on their behalf." - Geo. Orwell
Try this for reality: NOT ALL IRIDESCENT BLUE COPPER COINS BECAME THAT WAY NATURALLY - without the hand of man involved.
Anyone who denies this is well...not living in reality. That's a nice way of writing what I actually think of them.
Which of you copper color "ex-perts" is brave enough to go on record in this thread and post that in your opinion ALL IRIDESCENT BLUE COPPER COINS OCCURED NATURALLY.
Post-up, or get educated and quit your nonsense.
As I wrote before, some blue copper is natural and some is not. Nevertheless, it is up to the TPGS's and experienced dealers/collectors to set the standards for "market acceptable color." That said, none of us is perfect.
It's about 6:30 PM EST. Let's see how many hours before some brave copper "ex-pert" posts that in his opinion: ALL IRIDESCENT BLUE COPPER HAPPENED NATURALLY OVER TIME w/o the help of man.
So, following the diagram above, the OP's experiment failed because not all the coins changed color.
The answer to the question "Does MS70 turn copper blue" is therefore no.
When we ask "Under what conditions do copper coins turn blue with MS70" then we get to the truth. The only group of coins that turn blue are coins with some kind of coating on them prior to the treatment. This is a subset of all coins with coatings. Coating are dirt, debris, lacquer, shellac, etc.
Next question is "why do they turn blue". Here there is no straighforward answer because there are a multitude of coins with vastly different histories. I know that there were hoards of Proof Indian and Lincoln cents from 1878-1916 that are blue-toned. They came on the market in the late 1930's. These got blue toned by sitting in envelopes for over 50 years. Eliasberg had some in his collection.
The simple answer is that the toning was always there, but hidden by surface coatings. If you want to disagree, use the chart above and prove it. Just saying you saw the sun rise when the rooster crows isn't proof.
In the OP's example only the dark coin turned color. It had finger oil on it from circulation, as most copper does. We are not used to seeing the copper stripped of its naturally accrued oils. Bright red coins don't have debris on them, so there is no toning being covered up on them. Only the dark coin turned color. Is that toning artificial or natural? I would say it is unusual.
This poster did not even hit the ball. One reply, one swing and a miss...
@EagleEye said: "There are about the same variety of blue-toned coins as there are people who claim to be experts on this board. If you can say that all people who claim to be experts on this board really are, then...."
First of all, I have never read anything on CU where a member claimed to be an expert in anything. Some have come very close though. LOL. For what it's worth, I consider you to be as close to a true expert on Indian cents and their color as anyone in the world. Again, you have never shoved your expertise down anyone's craw.
That said, I appreciate you taking the time to comment on a well worn subject. Unfortunately, you have chosen not to accept my challenge (as no one is forced to). You have ONLY commented on "flat-earther's" and posted a nice flow chart.
Therefore, many thanks for weighing in and AFAIK and until you post what I asked or not - you are now in the Some Natural - Some Not Natural camp with me.
As I wrote, I don't believe there is any defender of blue copper who will say they ALL occurred naturally!
@EagleEye said:
So, following the diagram above, the OP's experiment failed because not all the coins changed color.
The answer to the question "Does MS70 turn copper blue" is therefore no.
When we ask "Under what conditions do copper coins turn blue with MS70" then we get to the truth. The only group of coins that turn blue are coins with some kind of coating on them prior to the treatment. This is a subset of all coins with coatings. Coating are dirt, debris, lacquer, shellac, etc.
Next question is "why do they turn blue". Here there is no straighforward answer because there are a multitude of coins with vastly different histories. I know that there were hoards of Proof Indian and Lincoln cents from 1878-1916 that are blue-toned. They came on the market in the late 1930's. These got blue toned by sitting in envelopes for over 50 years. Eliasberg had some in his collection.
The simple answer is that the toning was always there, but hidden by surface coatings. If you want to disagree, use the chart above and prove it. Just saying you saw the sun rise when the rooster crows isn't proof.
In the OP's example only the dark coin turned color. It had finger oil on it from circulation, as most copper does. We are not used to seeing the copper stripped of its naturally accrued oils. Bright red coins don't have debris on them, so there is no toning being covered up on them. Only the dark coin turned color. Is that toning artificial or natural? I would say it is unusual.
Now we are getting somewhere. Very well stated and very easy to understand. SOME COLOR CAN BE DESCRIBED AS BEING "UNUSUAL."
So, I have a copper coin with unknown history and surface residues possibly including dirt, oxidized copper haze (don't ask me - I'm not the chemist), old chemical residues, etc. This coin will basically remain in this condition - dull brown until the hand-of-man cleans it. I agree. Only the flat earth guy disagrees, right?
Now, I put a chemical on it and it MAY turn blue. It also may stay brown but it is now properly cleaned. Nothing left on the surface, no dirt, no haze, no oxidation, no nothing. The surface is "raw."
If coin stays brown it is out of the discussion.
If coin goes to blue, what exactly happened?
I say there was a REVERSIBLE chemical reaction that turned the coin blue. Others say the cleaning revealed the coin's true blue color. This does not compute. Hearsay has been posted in this thread that a blue coin turned back to brown all by itself. Did the little atoms in the atmosphere build up a new deposit on the coin's surface over time? If the true surface of a coin is blue how can the color be removed in seconds?
As I posted, I should happy if we can all agree that some blue color is natural and some is not as it will take some money and effort to run scientific tests on these coins and frankly some are so pretty I don't wish to know the truth!
@EagleEye said:
I say there is no chemical reaction.
A reversible chemical reaction? What are you smoking? In Denver already?
I'm no chemist but AFAIK, when I run water over a coin's surface a chemical reaction takes place. Furthermore, removing dirt with any type of thing other than a toothpick might also be considered some form of chemical reaction.
Turning a coin blue with a chemical is a reaction and turning a coin back to brown with a chemical is a reverse reaction.
Turning a coin blue with a chemical is a reaction and turning a coin back to brown with a chemical is a reverse reaction.
FIRST, ARE YOU ACTUALLY REACTING WITH THE METAL IN THE COIN OR SOMETHING ON THE SURFACE OF THE METAL? SECOND, HOW IT IS REVERSIBLE? CAN YOU SHOW US AN EQUILIBRIUM REACTION THAT CAN GO BOTH WAYS? OOPS, I FORGOT, YOU'RE NO CHEMIST.............
I've done this with dozens of Lincolns. I feel like I can spot MS70 from a mile away. I've also tested using several coin cleaners, DD, sulfur diluted in water and heat. I considered doing a write-up so people could spot the tells of these methods, but some of these turn Lincolns into market acceptable coins that I've seen in PCGS plastic. MS70 works best with already darkened coins -- at least RB but BN turns the most. It must be a reaction with contaminants already on the coin.
What is changing is the optical interference pattern which produces color. If a coating is on the surface the interference pattern is disturbed. Remove the coating and the color will likely come back. Not a chemical reaction.
@mach1ne said:
I've done this with dozens of Lincolns. I feel like I can spot MS70 from a mile away.MS70 works best with already darkened coins -- at least RB but BN turns the most. It must be a reaction with contaminants already on the coin.
What is does is strip off patina and organic material in most cases and then the coin surfaces underneath are revealed (it is a strong basic cleaning agent as we have seen in this and other threads). If said coin has colorful toning, you see the toning after removal of patina and gunk. Alternatively, in some cases, MS70 can indeed react with surface material on the coin and turn it blue. A very different look than toning where Cu is converted to an oxide or sulfide in a thin film. My experience is that the blue from reaction with contaminants is a volatile compound that goes away with time.
I'm no chemist but AFAIK, when I run water over a coin's surface a chemical reaction takes place. Furthermore, removing dirt with any type of thing other than a toothpick might also be considered some form of chemical reaction.
Turning a coin blue with a chemical is a reaction and turning a coin back to brown with a chemical is a reverse reaction.
Copper is not reactive with water, nor is bronze or copper-nickel. Copper is only reactive with contaminants in water. Copper does not seem to be reactive with MS70 either as evidenced by the OP's tests and as I stated in a previous post I cannnot make an RD coin turn blue with MS70........and I would LOVE to be able to do that as I collect IHH PR cents. All of my blue ones are PCGS certified. And I have purchased RAW blue IHC's and all have straight graded by PCGS. Interestingly, all RD RAW IHC PR coins that I have submitted to PCGS have graded code .91 Questionable Color. I would love to turn them blue with MS70. In fact.......hold on........I will treat one of my proof .91 coins with MS70 and will post the result in a day or two.
Reverse chemical reactions are most normally a result of the thermodynamic state of the material, i.e. the temperature of the material. Never caused by re-application of a chemical such as MS70. I am sure that coin doctors have "made" blue cents, but blue cents are only recently desireable. Rick Snow's research indicates troves of irredescent IHC's going back to the 1930's when they would not have been desireable.
This thread has morphed from the OP's test of MS70 on "copper" coins.
@OldIndianNutKase said: "Copper is not reactive with water, nor is bronze or copper-nickel. Copper is only reactive with contaminants in water."
So what? Keep this simple for all of the non-chemists here. I wish to become more informed. So if I run water on a copper coin and something on the coin reacts with something in the water you are saying that NO REACTION TAKES PLACE. I believe that's what I just read. Well, I'll confess to being extremely stupid as that does not make any sense to me.
@OINK Hey! I just figured out what OINK means at the end of your posts. I told you I was stupid.
OINK said: "Copper does not seem to be reactive with MS70 either as evidenced by the OP's tests and as I stated in a previous post I cannnot make an RD coin turn blue with MS70"
Agreed.
OINK said: "Reverse chemical reactions are most normally a result of the thermodynamic state of the material, i.e. the temperature of the material. Never caused by re-application of a chemical such as MS70."
Again, all I can say is I have turned a brown copper coin blue, back to brown, back to blue, back to brown and quit before I might ruin the coin. No trace of my test was visible when I stopped except the coin was beautiful. I call that reversing a reaction to a chemical. Again, out of my ignorance, I have probably used the wrong words. You see, when I do something and it changes, I call that reacting. An d if something in water can react with copper (your contention above), then I guess something in a chemical like MS-70 can also react with copper that is not red.
OINK said: "I am sure that coin doctors have "made" blue cents, but blue cents are only recently desireable. Rick Snow's research indicates troves of irredescent IHC's going back to the 1930's when they would not have been desireable. "
Great. This is the point I'm trying to make. You too have hit a home run. Some blue copper is "man-made."
@mach1ne said: "I've done this with dozens of Lincolns..." and "MS70 works best with already darkened coins -- at least RB but BN turns the most. It must be a reaction with contaminants already on the coin."
I agree, only I've done this with hundreds of copper coins of all ages, types, and countries. The only problem is that each time one turns blue it is a shock to me and I need to turn it brown again. LOL.
@spacehayduke said: "What is [it] does is strip off patina and organic material in most cases and then the coin surfaces underneath are revealed (it is a strong basic cleaning agent as we have seen in this and other threads). If said coin has colorful toning, you see the toning after removal of patina and gunk. Alternatively, in some cases, MS70 can indeed react with surface material on the coin and turn it blue. A very different look than toning where Cu is converted to an oxide or sulfide in a thin film. My experience is that the blue from reaction with contaminants is a volatile compound that goes away with time."
Great info! However, what I fail to see is when I have a lustrous BROWN AU to Unc copper coin and I soak it in acetone for several hours, and then Ultrasonic it, there should be no organic compounds left on the surface. And the coin is brown. Now I'll defer to the chemistry experts here. Why do SOME of these coins turn blue with nothing left on their surface? It never was blue until another chemical is applied. Where was the blue hiding? The other chemical MUST be leaving a residue of its own that refracts back as "blue." BUT THE COPPER METAL IS NEVER BLUE. Do I finally get it?
Great info! However, what I fail to see is when I have a lustrous BROWN AU to Unc copper coin and I soak it in acetone for several hours, and then Ultrasonic it, there should be no organic compounds left on the surface. And the coin is brown. Now I'll defer to the chemistry experts here. Why do SOME of these coins turn blue with nothing left on their surface? It never was blue until another chemical is applied. Where was the blue hiding? The other chemical MUST be leaving a residue of its own that refracts back as "blue." BUT THE COPPER METAL IS NEVER BLUE. Do I finally get it?
You sound like you have done all kinds of experiments. Please show your results using the scientific process in the chart above. You are assuming a chemical reaction. All we need to prove a chemical reaction is a repeatable experiment with the same outcome. Please show us.
I do not have the money to analyze a coin. I do know what happens when I conserve SOME coins - they turn blue and must be returned to brown.
As I wrote above, a professional grader is going to write a column in Numismatic News on this subject. In the last issue he mentioned in passing that at one time "blue" copper was considered to be an altered surface by a majority of numismatists.
I do not have a dog in the fight. I just want all the copper experts to do as you and OINK have done and admit that NOT ALL BLUE COLLORED COPPER IS NATURAL, that's all. I'm content to let you, auction companies, collectors, and TPGS decide AT or NT.
Thanks for the humor! The older and wiser chimps use rocks to crack the nuts. While I'm the little chimp using a log, I consider you an older and wiser numismatist. Save me some time here. Since I sadly misquoted you earlier, I don't wish to do it again.
Do you believe that all "blue" copper is a natural occurrence or that some is man made?
Thanks for the humor! The older and wiser chimps use rocks to crack the nuts. While I'm the little chimp using a log, I consider you an older and wiser numismatist. Save me some time here. Since I sadly misquoted you earlier, I don't wish to do it again.
Do you believe that all "blue" copper is a natural occurrence or that some is man made?
I can't see where brg ever said that all blue copper is natural - this is the 3rd time you have tried to poke him on this - why?
@spacehayduke writes: "I can't see where brg ever said that all blue copper is natural - this is the 3rd time you have tried to poke him on this - why?"
I think it is because I can read "between the lines." The member you asked me about posted this on page one with regard to another member's opinion:
brg5658 Posts: 2,109 ✭✭✭
July 25, 2017 12:02PM @You said:
Blue is not a possible natural color for business strike copper to tone. It is caused by the MS70, not revealed by it. The blue color can be removed.
Absolute nonsense.
@brg5658 thinks this post is nonsense. While I agree with brg 100% concerning the first sentence in @You's post which IS UNINFORMED NONSENSE, the rest is not. Unfortunately, I misquoted brg in a post. Now I wish to set my mind at ease for that mistake and learn if he thinks "blue" copper is AT, NT, or it can be either, depending on the coin. I value his opinion. This could have been settled yesterday but apparently the member wishes to post funny images while keeping me twisting in the wind and filled with remorse. I guess I deserve to pound nuts for what I posted about him.
So to quote Rick, "Now, by now we all should know the true answer as to why the copper coins are blue is that the chemical didn't produce it – the chemical uncovered what was already there." Then why doesn't this happen if acetone is used? It's only for MS70, but acetone also reveals what is underneath the surface. And why is it that the blue tone from MS70 usage can easily be removed with a variety of methods? It's obviously produced by the MS70 and not just being uncovered. That spot of nonsense makes me lose faith in the rest of Rick's post.
You all keep mentioning the "scientific process." The thing is, it is much more difficult to prove that something exists than that it doesn't exist. I can't prove that natural blue business strike (by which I mean not proof or made from proof-like dies or with mirror fields, just plain old circulation coinage) copper cannot exist, but it is possible to prove that it does. I have not been presented with any evidence that it does exist, but have seen hundreds of examples of blue circulation strike copper on the market that have clearly been MS70ed. Therefore I will continue to believe that it doesn't exist.
"I have not been presented with any evidence that it does exist, but have seen hundreds of examples of blue circulation strike copper on the market that have clearly been MS70ed. Therefore I will continue to believe that it DOESEN'T exist."
"I have not been presented with any evidence that it does exist, but have seen hundreds of examples of blue circulation strike copper on the market that have clearly been MS70ed. Therefore I will continue to believe that it DOESEN'T exist."
It's simple. Here's a comparative example: an atheist does not believe in a god because he has not been presented with any evidence that a god exists. The burden of proof lies on the person who says that "x" exists, whether it be a god or natural blue toning. There is no reason to believe something exists if there is no evidence that it exists.
I found a circulated roll of IHC's that I have owned for over 60 years. I searched that roll for any coins that have any blue or green coloration and found the following examples:
The 1897 (lower left) seemed to be the best candidate for an MS70 trial which consisted of application 20-30 seconds followed by a warm soap water bath. Before MS70 the obverse:
And after the MS70 dip and soap water bath it looked like this:
I find this demonstrates that clue/green toning can exist on the coin before the dip and that after that dip the color just becomes more visible. From this I conclude that MS70 did not create the color, but it did remove some of the dingy brown color that lies over the top of the color toning.
I appologize for the phone pictures, but I was anxious to test the theory rather than showcase my photographic skills.
@OldIndianNutKase said:
I found a circulated roll of IHC's that I have owned for over 60 years. I searched that roll for any coins that have any blue or green coloration and found the following examples:
The 1897 (lower left) seemed to be the best candidate for an MS70 trial which consisted of application 20-30 seconds followed by a warm soap water bath. Before MS70 the obverse:
And after the MS70 dip and soap water bath it looked like this:
I find this demonstrates that clue/green toning can exist on the coin before the dip and that after that dip the color just becomes more visible. From this I conclude that MS70 did not create the color, but it did remove some of the dingy brown color that lies over the top of the color toning.
I appologize for the phone pictures, but I was anxious to test the theory rather than showcase my photographic skills.
OINK
All of those coins look like they have environmental damage. That color doesn't look like any sort of toning, artificial or not, but more like PVC or something.
Here's something of interest: brass seems to be extremely reactive to MS70 in this way, much more so than copper. If you MS70 brass tokens (ex. Civil war tokens), they seem to uniformly turn all sorts of red, blue, and purple colors (that can EASILY be removed with a simple bit of olive oil, just like with all of the copper examples, so it is NOT just underneath the surface. If it were, olive oil would have no effect). Possibly it is the presence of the non-copper elements that causes the reaction? Even copper cents aren't completely copper.
All of those coins look like they have environmental damage. That color doesn't look like any sort of toning, artificial or not, but more like PVC or something.
What you seem to not understand is that copper tones blue naturally. MS70 is not the source of the blue toning on copper or bronze coins. In my business I have bought thousands of pounds of copper pipe, and over time it will develop blue "toning" or environmental damage if you insist. It will not stay red. Although I agree with most everything Rick Snow's research has shown, the blue coating is caused by a chemical reaction. It is not a coating. It's chemical composition is CuSO3 or CuSO4 which are both known to have bluish or greenish coloration. Applying MS70 or acetone is not reactive with either of the copper sulfate compounds previously cited. But they will remove the overlying film that hides the color of the sulfates. This was clearly demonstrated by my recent experiment.
You continue to post your opinions which have no factual basis from a scientific point of view. You are really a very poor representative of those that think blue toning on IHC's is the result of MS70. You might want to be more careful before you end up being sued by the manufacturer of MS70 for slander or libel. If you have a legitimate case, you need to present it on a much more technically sound basis.
You might want to be more careful before you end up being sued by the manufacturer of MS70 for slander or libel. If you have a legitimate case, you need to present it on a much more technically sound basis.
Hahaha what? I am not publishing anything I'm saying here. That's not how it works and is pretty dumb thing to say.
@OldIndianNutKase said:
What you seem to not understand is that copper tones blue naturally. MS70 is not the source of the blue toning on copper or bronze coins. In my business I have bought thousands of pounds of copper pipe, and over time it will develop blue "toning" or environmental damage if you insist. It will not stay red.
Oxidization on copper coins turns them brown, not blue or green (that's why older copper is red-brown or brown). They are not pure copper.
@OldIndianNutKase said:
Applying MS70 or acetone is not reactive with either of the copper sulfate compounds previously cited. But they will remove the overlying film that hides the color of the sulfates. This was clearly demonstrated by my recent experiment.
Your "experiment" is a bunch of bogus because those coins did not have natural color in the first place. Anyone can see that there was environmental damage in the form of PVC or some other substance beforehand. I do not mean toning by "environmental damage," I mean environmental damage that would lead to those coins not being slabbed.
Copper oxidizes green/ aqua for the most part, look at the statue of liberty, which is/was the world's largest copper statue. What color does it look like? It deffently not brown.
Now add in some zinc and other environmental gasses and anything is possible. Most of those coins have either been circulated or stored in different conditions and could have picked up any number of contaminants I guess I believe what has already been said. Not all blue copper is ms70d, that's just a silly statement.
Walker Proof Digital Album Fellas, leave the tight pants to the ladies. If I can count the coins in your pockets you better use them to call a tailor. Stay thirsty my friends......
"I have not been presented with any evidence that it does exist, but have seen hundreds of examples of blue circulation strike copper on the market that have clearly been MS70ed. Therefore I will continue to believe that it DOESEN'T exist."
It's simple. Here's a comparative example: an atheist does not believe in a god because he has not been presented with any evidence that a god exists. The burden of proof lies on the person who says that "x" exists, whether it be a god or natural blue toning. There is no reason to believe something exists if there is no evidence that it exists.
Oh, All you needed to say was you do not believe a copper coin can turn shades of blue naturally! Sorry you believe that as you are uninformed. Perhaps one day you will have a chance to find one for yourself. An easy way is to store some AU copper Lincolns in various locations in contact with different materials. Do you have an attic, cedar chest, window sill? Leave a dozen or so around with 2-3 (in contact with different material) in the same location. Wait a few years...LOL.
Comments
Well... this is refreshing..... Some people actually doing real experiments.... Though I am not sure they have been done under controlled conditions, at least we may be stepping beyond the state of 'uninformed opinion'.
Rick Snow has posted in depth information on the 'blue' copper phenomenon..... those who missed it, should look it up. Certainly do some experimentation -valuable experience. I spent a couple of years doing experiments with silver tarnish. For those too busy to do their own tests, for copper, please read Rick's treatise on the subject. Cheers, RickO
I think there are about eight different dies/die states for this very tricky date, and business strike were obviously struck from proof dies. Are @You an EAC guy?
lol'd
I see the saga of market acceptable blue copper continues.
Once you do it yourself and see it with your own two eyes, it's hard to continue to deny....or not, apparently.
The more things change, the more they stay the same.
Link to Rick's thread. A few here could learn a thing or two about reality.
https://forums.collectors.com/discussion/977997/the-sun-and-the-blue-toned-rooster/p1
-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-
My sets: [280+ horse coins] :: [France Sowers] :: [Colorful world copper] :: [Beautiful world coins]
-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-
Try this for reality: NOT ALL IRIDESCENT BLUE COPPER COINS BECAME THAT WAY NATURALLY - without the hand of man involved.
Anyone who denies this is well...not living in reality. That's a nice way of writing what I actually think of them.
Which of you copper color "ex-perts" is brave enough to go on record in this thread and post that in your opinion ALL IRIDESCENT BLUE COPPER COINS OCCURED NATURALLY.
Post-up, or get educated and quit your nonsense.
As I wrote before, some blue copper is natural and some is not. Nevertheless, it is up to the TPGS's and experienced dealers/collectors to set the standards for "market acceptable color." That said, none of us is perfect.
It's about 6:30 PM EST. Let's see how many hours before some brave copper "ex-pert" posts that in his opinion: ALL IRIDESCENT BLUE COPPER HAPPENED NATURALLY OVER TIME w/o the help of man.
There are about the same variety of blue-toned coins as there are people who claim to be experts on this board.
If you can say that all people who claim to be experts on this board really are, then....
The scientific method.
If you don't follow this you are a flat-earther.
So, following the diagram above, the OP's experiment failed because not all the coins changed color.
The answer to the question "Does MS70 turn copper blue" is therefore no.
When we ask "Under what conditions do copper coins turn blue with MS70" then we get to the truth. The only group of coins that turn blue are coins with some kind of coating on them prior to the treatment. This is a subset of all coins with coatings. Coating are dirt, debris, lacquer, shellac, etc.
Next question is "why do they turn blue". Here there is no straighforward answer because there are a multitude of coins with vastly different histories. I know that there were hoards of Proof Indian and Lincoln cents from 1878-1916 that are blue-toned. They came on the market in the late 1930's. These got blue toned by sitting in envelopes for over 50 years. Eliasberg had some in his collection.
The simple answer is that the toning was always there, but hidden by surface coatings. If you want to disagree, use the chart above and prove it. Just saying you saw the sun rise when the rooster crows isn't proof.
In the OP's example only the dark coin turned color. It had finger oil on it from circulation, as most copper does. We are not used to seeing the copper stripped of its naturally accrued oils. Bright red coins don't have debris on them, so there is no toning being covered up on them. Only the dark coin turned color. Is that toning artificial or natural? I would say it is unusual.
This poster did not even hit the ball. One reply, one swing and a miss...
@EagleEye said: "There are about the same variety of blue-toned coins as there are people who claim to be experts on this board. If you can say that all people who claim to be experts on this board really are, then...."
First of all, I have never read anything on CU where a member claimed to be an expert in anything. Some have come very close though. LOL. For what it's worth, I consider you to be as close to a true expert on Indian cents and their color as anyone in the world. Again, you have never shoved your expertise down anyone's craw.
That said, I appreciate you taking the time to comment on a well worn subject. Unfortunately, you have chosen not to accept my challenge (as no one is forced to). You have ONLY commented on "flat-earther's" and posted a nice flow chart.
Therefore, many thanks for weighing in and AFAIK and until you post what I asked or not - you are now in the Some Natural - Some Not Natural camp with me.
As I wrote, I don't believe there is any defender of blue copper who will say they ALL occurred naturally!
Come on folks...almost an hour has passed.
I think your last post was being written while I posted again. You need to ask a less pointed question to get to an answer.
Now we are getting somewhere. Very well stated and very easy to understand. SOME COLOR CAN BE DESCRIBED AS BEING "UNUSUAL."
So, I have a copper coin with unknown history and surface residues possibly including dirt, oxidized copper haze (don't ask me - I'm not the chemist), old chemical residues, etc. This coin will basically remain in this condition - dull brown until the hand-of-man cleans it. I agree. Only the flat earth guy disagrees, right?
Now, I put a chemical on it and it MAY turn blue. It also may stay brown but it is now properly cleaned. Nothing left on the surface, no dirt, no haze, no oxidation, no nothing. The surface is "raw."
If coin stays brown it is out of the discussion.
If coin goes to blue, what exactly happened?
I say there was a REVERSIBLE chemical reaction that turned the coin blue. Others say the cleaning revealed the coin's true blue color. This does not compute. Hearsay has been posted in this thread that a blue coin turned back to brown all by itself. Did the little atoms in the atmosphere build up a new deposit on the coin's surface over time? If the true surface of a coin is blue how can the color be removed in seconds?
As I posted, I should happy if we can all agree that some blue color is natural and some is not as it will take some money and effort to run scientific tests on these coins and frankly some are so pretty I don't wish to know the truth!
@EagleEye said: "As I wrote, I don't believe there is any defender of blue copper who will say they ALL occurred naturally!
Thank you. That is a HOME RUN!
Now, how about it @brg5658? Care to take a swing and defend your previous post?
I say there is no chemical reaction.
A reversible chemical reaction? What are you smoking? In Denver already?
First, thanks for your response, I appreciate the more thorough explanation of my experiment.
Second, it's been a while since I've taken Chemistry, but...https://en.wikipedia.org/wiki/Reversible_reaction. Look under the history tab.
I'm no chemist but AFAIK, when I run water over a coin's surface a chemical reaction takes place. Furthermore, removing dirt with any type of thing other than a toothpick might also be considered some form of chemical reaction.
Turning a coin blue with a chemical is a reaction and turning a coin back to brown with a chemical is a reverse reaction.
Shucks, I thought we getting somewhere.
YUP WE GOT THAT
FIRST, ARE YOU ACTUALLY REACTING WITH THE METAL IN THE COIN OR SOMETHING ON THE SURFACE OF THE METAL? SECOND, HOW IT IS REVERSIBLE? CAN YOU SHOW US AN EQUILIBRIUM REACTION THAT CAN GO BOTH WAYS? OOPS, I FORGOT, YOU'RE NO CHEMIST.............
I've done this with dozens of Lincolns. I feel like I can spot MS70 from a mile away. I've also tested using several coin cleaners, DD, sulfur diluted in water and heat. I considered doing a write-up so people could spot the tells of these methods, but some of these turn Lincolns into market acceptable coins that I've seen in PCGS plastic. MS70 works best with already darkened coins -- at least RB but BN turns the most. It must be a reaction with contaminants already on the coin.
Check out my iPhone app SlabReader!
What is changing is the optical interference pattern which produces color. If a coating is on the surface the interference pattern is disturbed. Remove the coating and the color will likely come back. Not a chemical reaction.
What is does is strip off patina and organic material in most cases and then the coin surfaces underneath are revealed (it is a strong basic cleaning agent as we have seen in this and other threads). If said coin has colorful toning, you see the toning after removal of patina and gunk. Alternatively, in some cases, MS70 can indeed react with surface material on the coin and turn it blue. A very different look than toning where Cu is converted to an oxide or sulfide in a thin film. My experience is that the blue from reaction with contaminants is a volatile compound that goes away with time.
Best, SH
Copper is not reactive with water, nor is bronze or copper-nickel. Copper is only reactive with contaminants in water. Copper does not seem to be reactive with MS70 either as evidenced by the OP's tests and as I stated in a previous post I cannnot make an RD coin turn blue with MS70........and I would LOVE to be able to do that as I collect IHH PR cents. All of my blue ones are PCGS certified. And I have purchased RAW blue IHC's and all have straight graded by PCGS. Interestingly, all RD RAW IHC PR coins that I have submitted to PCGS have graded code .91 Questionable Color. I would love to turn them blue with MS70. In fact.......hold on........I will treat one of my proof .91 coins with MS70 and will post the result in a day or two.
Reverse chemical reactions are most normally a result of the thermodynamic state of the material, i.e. the temperature of the material. Never caused by re-application of a chemical such as MS70. I am sure that coin doctors have "made" blue cents, but blue cents are only recently desireable. Rick Snow's research indicates troves of irredescent IHC's going back to the 1930's when they would not have been desireable.
This thread has morphed from the OP's test of MS70 on "copper" coins.
OINK
@OldIndianNutKase said: "Copper is not reactive with water, nor is bronze or copper-nickel. Copper is only reactive with contaminants in water."
So what? Keep this simple for all of the non-chemists here. I wish to become more informed. So if I run water on a copper coin and something on the coin reacts with something in the water you are saying that NO REACTION TAKES PLACE. I believe that's what I just read. Well, I'll confess to being extremely stupid as that does not make any sense to me.
@OINK Hey! I just figured out what OINK means at the end of your posts. I told you I was stupid.
OINK said: "Copper does not seem to be reactive with MS70 either as evidenced by the OP's tests and as I stated in a previous post I cannnot make an RD coin turn blue with MS70"
Agreed.
OINK said: "Reverse chemical reactions are most normally a result of the thermodynamic state of the material, i.e. the temperature of the material. Never caused by re-application of a chemical such as MS70."
Again, all I can say is I have turned a brown copper coin blue, back to brown, back to blue, back to brown and quit before I might ruin the coin. No trace of my test was visible when I stopped except the coin was beautiful. I call that reversing a reaction to a chemical. Again, out of my ignorance, I have probably used the wrong words. You see, when I do something and it changes, I call that reacting. An d if something in water can react with copper (your contention above), then I guess something in a chemical like MS-70 can also react with copper that is not red.
OINK said: "I am sure that coin doctors have "made" blue cents, but blue cents are only recently desireable. Rick Snow's research indicates troves of irredescent IHC's going back to the 1930's when they would not have been desireable. "
Great. This is the point I'm trying to make. You too have hit a home run. Some blue copper is "man-made."
@mach1ne said: "I've done this with dozens of Lincolns..." and "MS70 works best with already darkened coins -- at least RB but BN turns the most. It must be a reaction with contaminants already on the coin."
I agree, only I've done this with hundreds of copper coins of all ages, types, and countries. The only problem is that each time one turns blue it is a shock to me and I need to turn it brown again. LOL.
@spacehayduke said: "What is [it] does is strip off patina and organic material in most cases and then the coin surfaces underneath are revealed (it is a strong basic cleaning agent as we have seen in this and other threads). If said coin has colorful toning, you see the toning after removal of patina and gunk. Alternatively, in some cases, MS70 can indeed react with surface material on the coin and turn it blue. A very different look than toning where Cu is converted to an oxide or sulfide in a thin film. My experience is that the blue from reaction with contaminants is a volatile compound that goes away with time."
Great info! However, what I fail to see is when I have a lustrous BROWN AU to Unc copper coin and I soak it in acetone for several hours, and then Ultrasonic it, there should be no organic compounds left on the surface. And the coin is brown. Now I'll defer to the chemistry experts here. Why do SOME of these coins turn blue with nothing left on their surface? It never was blue until another chemical is applied. Where was the blue hiding? The other chemical MUST be leaving a residue of its own that refracts back as "blue." BUT THE COPPER METAL IS NEVER BLUE. Do I finally get it?
You sound like you have done all kinds of experiments. Please show your results using the scientific process in the chart above. You are assuming a chemical reaction. All we need to prove a chemical reaction is a repeatable experiment with the same outcome. Please show us.
I do not have the money to analyze a coin. I do know what happens when I conserve SOME coins - they turn blue and must be returned to brown.
As I wrote above, a professional grader is going to write a column in Numismatic News on this subject. In the last issue he mentioned in passing that at one time "blue" copper was considered to be an altered surface by a majority of numismatists.
I do not have a dog in the fight. I just want all the copper experts to do as you and OINK have done and admit that NOT ALL BLUE COLLORED COPPER IS NATURAL, that's all. I'm content to let you, auction companies, collectors, and TPGS decide AT or NT.
-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-
My sets: [280+ horse coins] :: [France Sowers] :: [Colorful world copper] :: [Beautiful world coins]
-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-
@brg5658
Thanks for the humor! The older and wiser chimps use rocks to crack the nuts. While I'm the little chimp using a log, I consider you an older and wiser numismatist. Save me some time here. Since I sadly misquoted you earlier, I don't wish to do it again.
Do you believe that all "blue" copper is a natural occurrence or that some is man made?
I can't see where brg ever said that all blue copper is natural - this is the 3rd time you have tried to poke him on this - why?
@spacehayduke writes: "I can't see where brg ever said that all blue copper is natural - this is the 3rd time you have tried to poke him on this - why?"
I think it is because I can read "between the lines." The member you asked me about posted this on page one with regard to another member's opinion:
brg5658 Posts: 2,109 ✭✭✭
July 25, 2017 12:02PM
@You said:
Blue is not a possible natural color for business strike copper to tone. It is caused by the MS70, not revealed by it. The blue color can be removed.
Absolute nonsense.
@brg5658 thinks this post is nonsense. While I agree with brg 100% concerning the first sentence in @You's post which IS UNINFORMED NONSENSE, the rest is not. Unfortunately, I misquoted brg in a post. Now I wish to set my mind at ease for that mistake and learn if he thinks "blue" copper is AT, NT, or it can be either, depending on the coin. I value his opinion. This could have been settled yesterday but apparently the member wishes to post funny images while keeping me twisting in the wind and filled with remorse. I guess I deserve to pound nuts for what I posted about him.
Rick is specifically discussing proof copper.
Gobrecht's Engraved Mature Head Large Cent Model
https://www.instagram.com/rexrarities/?hl=en
Not taking any sides here, but just thought I might point that out.
Gobrecht's Engraved Mature Head Large Cent Model
https://www.instagram.com/rexrarities/?hl=en
Thanks, MS and Proof copper can turn blue under the right conditions. I believe some of it is natural - especially on the Proofs.
So to quote Rick, "Now, by now we all should know the true answer as to why the copper coins are blue is that the chemical didn't produce it – the chemical uncovered what was already there." Then why doesn't this happen if acetone is used? It's only for MS70, but acetone also reveals what is underneath the surface. And why is it that the blue tone from MS70 usage can easily be removed with a variety of methods? It's obviously produced by the MS70 and not just being uncovered. That spot of nonsense makes me lose faith in the rest of Rick's post.
You all keep mentioning the "scientific process." The thing is, it is much more difficult to prove that something exists than that it doesn't exist. I can't prove that natural blue business strike (by which I mean not proof or made from proof-like dies or with mirror fields, just plain old circulation coinage) copper cannot exist, but it is possible to prove that it does. I have not been presented with any evidence that it does exist, but have seen hundreds of examples of blue circulation strike copper on the market that have clearly been MS70ed. Therefore I will continue to believe that it doesn't exist.
@You
You lost me here...
"I have not been presented with any evidence that it does exist, but have seen hundreds of examples of blue circulation strike copper on the market that have clearly been MS70ed. Therefore I will continue to believe that it DOESEN'T exist."
It's simple. Here's a comparative example: an atheist does not believe in a god because he has not been presented with any evidence that a god exists. The burden of proof lies on the person who says that "x" exists, whether it be a god or natural blue toning. There is no reason to believe something exists if there is no evidence that it exists.
I found a circulated roll of IHC's that I have owned for over 60 years. I searched that roll for any coins that have any blue or green coloration and found the following examples:



The 1897 (lower left) seemed to be the best candidate for an MS70 trial which consisted of application 20-30 seconds followed by a warm soap water bath. Before MS70 the obverse:
And after the MS70 dip and soap water bath it looked like this:
I find this demonstrates that clue/green toning can exist on the coin before the dip and that after that dip the color just becomes more visible. From this I conclude that MS70 did not create the color, but it did remove some of the dingy brown color that lies over the top of the color toning.
I appologize for the phone pictures, but I was anxious to test the theory rather than showcase my photographic skills.
OINK
All of those coins look like they have environmental damage. That color doesn't look like any sort of toning, artificial or not, but more like PVC or something.
Here's something of interest: brass seems to be extremely reactive to MS70 in this way, much more so than copper. If you MS70 brass tokens (ex. Civil war tokens), they seem to uniformly turn all sorts of red, blue, and purple colors (that can EASILY be removed with a simple bit of olive oil, just like with all of the copper examples, so it is NOT just underneath the surface. If it were, olive oil would have no effect). Possibly it is the presence of the non-copper elements that causes the reaction? Even copper cents aren't completely copper.
What you seem to not understand is that copper tones blue naturally. MS70 is not the source of the blue toning on copper or bronze coins. In my business I have bought thousands of pounds of copper pipe, and over time it will develop blue "toning" or environmental damage if you insist. It will not stay red. Although I agree with most everything Rick Snow's research has shown, the blue coating is caused by a chemical reaction. It is not a coating. It's chemical composition is CuSO3 or CuSO4 which are both known to have bluish or greenish coloration. Applying MS70 or acetone is not reactive with either of the copper sulfate compounds previously cited. But they will remove the overlying film that hides the color of the sulfates. This was clearly demonstrated by my recent experiment.
You continue to post your opinions which have no factual basis from a scientific point of view. You are really a very poor representative of those that think blue toning on IHC's is the result of MS70. You might want to be more careful before you end up being sued by the manufacturer of MS70 for slander or libel. If you have a legitimate case, you need to present it on a much more technically sound basis.
OINK
All this talk about turning copper blue...pink is the next big rage can you all do that?
Hahaha what? I am not publishing anything I'm saying here. That's not how it works and is pretty dumb thing to say.
Oxidization on copper coins turns them brown, not blue or green (that's why older copper is red-brown or brown). They are not pure copper.
Your "experiment" is a bunch of bogus because those coins did not have natural color in the first place. Anyone can see that there was environmental damage in the form of PVC or some other substance beforehand. I do not mean toning by "environmental damage," I mean environmental damage that would lead to those coins not being slabbed.
Pink is very nice too.........

and another copper coin that is mostly pink:
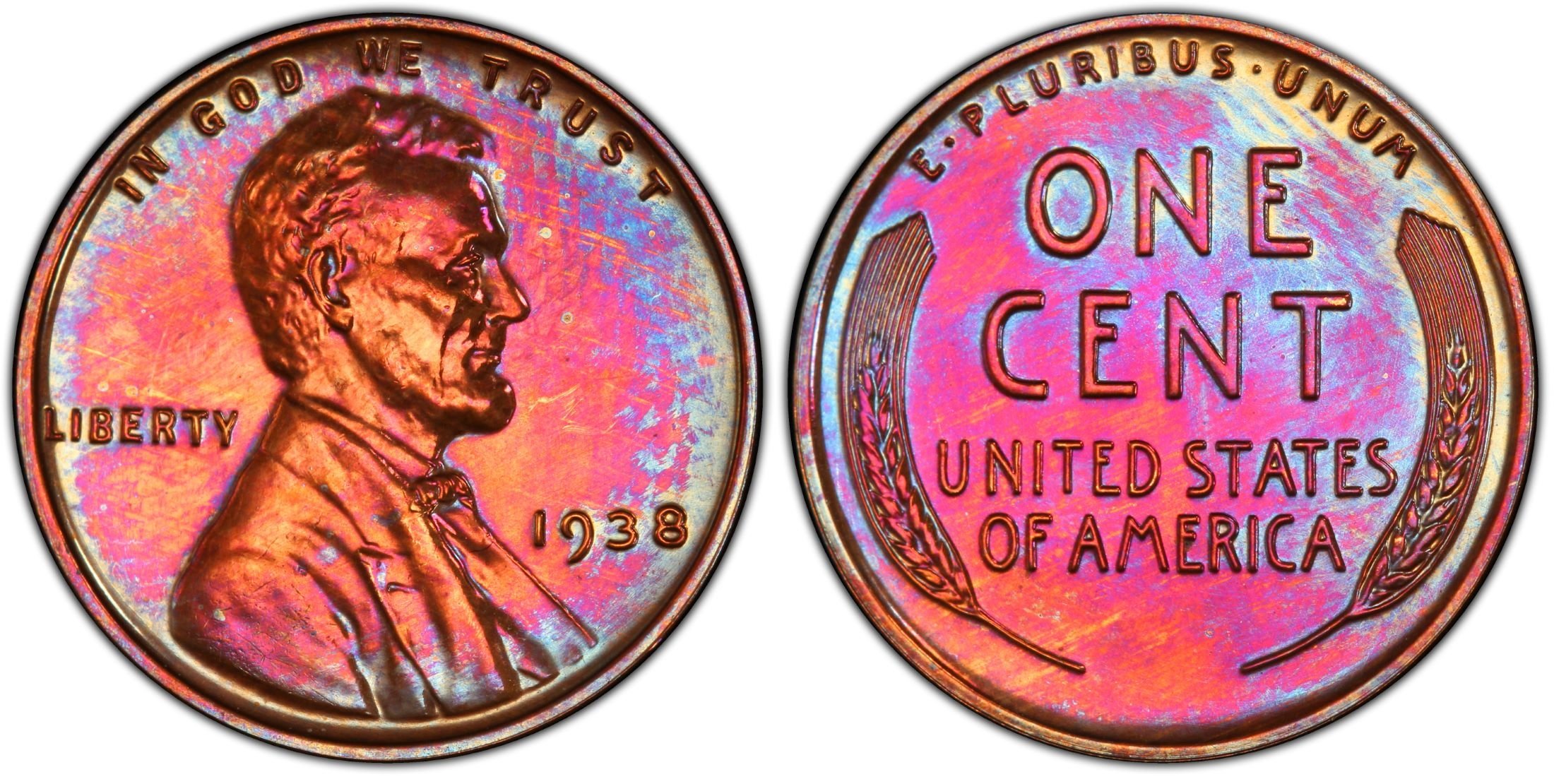
Copper does not naturally tone brown. The brown is surface contaminents.
No it's not.
Anyway, I'm getting bored of this. Cya.
You will not be missed.......
OINK
Copper oxidizes green/ aqua for the most part, look at the statue of liberty, which is/was the world's largest copper statue. What color does it look like? It deffently not brown.
Now add in some zinc and other environmental gasses and anything is possible. Most of those coins have either been circulated or stored in different conditions and could have picked up any number of contaminants I guess I believe what has already been said. Not all blue copper is ms70d, that's just a silly statement.
eBay ID-bruceshort978
Successful BST:here and ATS, bumanchu, wdrob, hashtag, KeeNoooo, mikej61, Yonico, Meltdown, BAJJERFAN, Excaliber, lordmarcovan, cucamongacoin, robkool, bradyc, tonedcointrader, mumu, Windycity, astrotrain, tizofthe, overdate, rwyarmch, mkman123, Timbuk3,GBurger717, airplanenut, coinkid855 ,illini420, michaeldixon, Weiss, Morpheus, Deepcoin, Collectorcoins, AUandAG, D.Schwager.
EACer cracking a slab?
mark
Fellas, leave the tight pants to the ladies. If I can count the coins in your pockets you better use them to call a tailor. Stay thirsty my friends......
Read the entire thread, and you will see otherwise.
Just thought I might point that out.
-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-
My sets: [280+ horse coins] :: [France Sowers] :: [Colorful world copper] :: [Beautiful world coins]
-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-~-
Oh, All you needed to say was you do not believe a copper coin can turn shades of blue naturally! Sorry you believe that as you are uninformed. Perhaps one day you will have a chance to find one for yourself. An easy way is to store some AU copper Lincolns in various locations in contact with different materials. Do you have an attic, cedar chest, window sill? Leave a dozen or so around with 2-3 (in contact with different material) in the same location. Wait a few years...LOL.