Is there a non-destructive way to differentiate copper / zinc cents without weighing them?
 seanq
Posts: 8,767 ✭✭✭✭✭
seanq
Posts: 8,767 ✭✭✭✭✭
Wondering if there is a non-destructive way to differentiate 1982 cent by composition without knowing the weight.
I recently won this clipped '82 cent at auction, the clip is large enough that the weight alone does not immediately indicate the composition. I ended up playing with the photo and Googling some geometry formulas to estimate the percentage of the clip, see below.
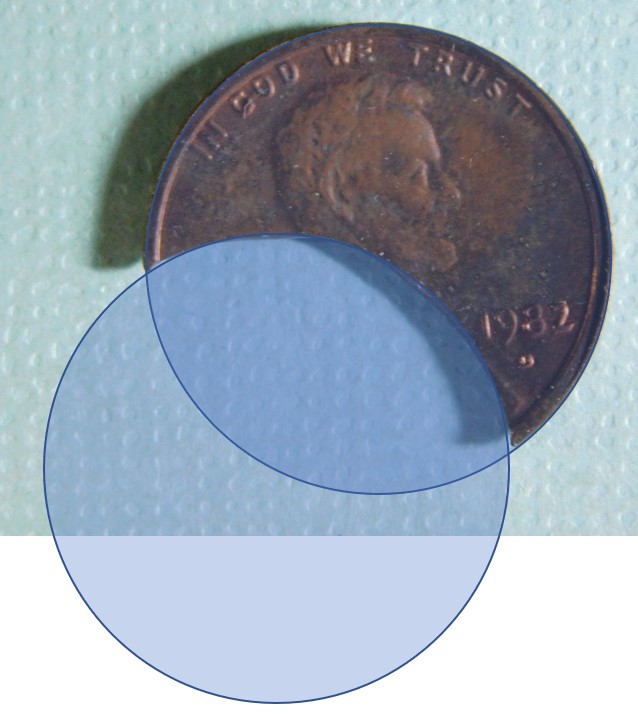
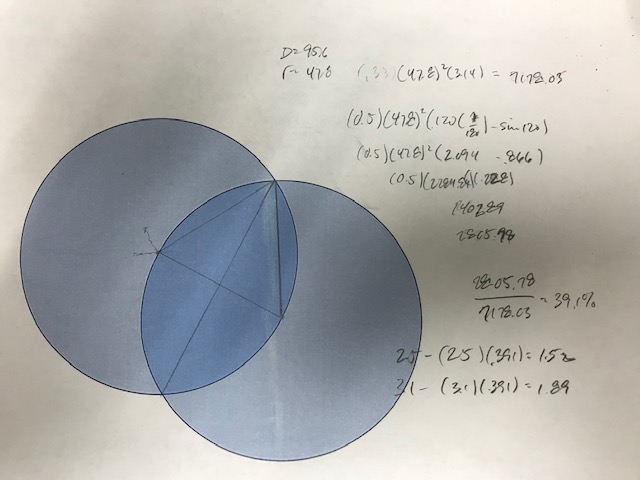
(Note that while I wrote the last calculations as (2.5) (.391), what I actually did was multiply by (.609), which would give the weight of the remaining planchet versus the weight of the missing area)
Based on the seller's reported weight (1.5g) and a 39% clip by volume, I believe this coin is on a zinc planchet, which should weigh 2.5g without the error. It's not that I don't trust my math, but I would like a more definitive way to confirm this without having to damage the edge of the coin.
Thanks,
Sean Reynolds
"Keep in mind that most of what passes as numismatic information is no more than tested opinion at best, and marketing blather at worst. However, I try to choose my words carefully, since I know that you guys are always watching." - Joe O'Connor
Comments
Toss them against an anvil. Bronze will "ting" and zinc will "thunk."
(If you have the clip authenticated the TPG might include composition on the label -- why not ask PCGS?)
Trig! Nice!
Now the metallurgy...
Edit to add: Or, acoustical physics. 😀
Did you try a specific gravity test? Roger's method will work also... Cheers, RickO
Cheers, RickO
Bronze cents ring when you flip them (i.e., strike with thumbnail sending airborne), Zincolns don't.
Keeper of the VAM Catalog • Professional Coin Imaging • Prime Number Set • World Coins in Early America • British Trade Dollars • Variety Attribution
The density could also be calculated, but for this light an item measurements must be extremely accurate.
XRF measurement on the clip will also reveal the alloy; of course the exposed clip should also show the zinc core, so there would be no need for testing. A bronze cent is homogeneous; a zinc cent is layered.
XRF is probably your best bet without using weight.
IMO you can tell (not with 100% certainty obviously) based off of the relief as well. Zinc cents are usually much lower relief than copper and from what I see the one pictured above looks like zinc.
Roger,
The core will not show on a genuine zinc clip, because the planchets are plated after they are punched. The seller confirmed there is copper on the inside of the clip, which only reinforces that the coin is a Mint error.
Sean Reynolds
"Keep in mind that most of what passes as numismatic information is no more than tested opinion at best, and marketing blather at worst. However, I try to choose my words carefully, since I know that you guys are always watching." - Joe O'Connor
Sean, Thanks! I had erroneously assumed the strip was plated as was done for 1943 cents.
Do the specific gravity test. That will give you the density.
Please show the picture without the overlay. There does not seem to be much distortion during the strike. If it were an incomplete clip on a zinc planchet that broke apart after the strike the cut arc would not be plated.
XRF penetrates slightly below the surface of the sample. The penetration will go below the standard copper plating thickness and into the bulk zinc. If you readout shows a trace or les of Zn, then it would be a copper cent. Any appreciable amount of Zn would indicate a Copper planted zinc cent.
I used to use a simple ring test when the coins first came out in 1982. Balance the coin in question on the tip of your finger (pinkie worked best for me). Then gently tap the edge of the coin with a pre-1982 copper cent. If the test coin rings, it's copper, if it makes a dull thus, it's zinc. Much safer than tossing on a hard surface. You may have to try it with a couple to get the hang of it, but it's not hard. Will the clip affect the ringing? I don't think so, but it's something to consider
Tom, here are the seller's pictures, I do not have the coin in hand, it is out for delivery.
Sean Reynolds
"Keep in mind that most of what passes as numismatic information is no more than tested opinion at best, and marketing blather at worst. However, I try to choose my words carefully, since I know that you guys are always watching." - Joe O'Connor
You can buy a 'cheap' coin comparitor such as used in vending machines........I sorted 100,000's of cents that way.
I built my own
 https://youtu.be/pANmastNB24
https://youtu.be/pANmastNB24
Successful transactions with : MICHAELDIXON, Manorcourtman, Bochiman, bolivarshagnasty, AUandAG, onlyroosies, chumley, Weiss, jdimmick, BAJJERFAN, gene1978, TJM965, Smittys, GRANDAM, JTHawaii, mainejoe, softparade, derryb, Ricko
Bad transactions with : nobody to date
I hate math, but... disregarding any +/- tolerances, if you say the clip is 1.5 g
Zinc = 2.5, if clip is 1.5 that is 60% of the planchet, meaning 40% clip
Copper = 3.11, if clip is 1.5 that is ~48% of the planchet, meaning 52% clip
Based on your overlays and eyeball % of 39% - given the difference between 40% (zinc) and 52% (copper) - I'd go with Zinc.
"You Suck Award" - February, 2015
Discoverer of 1919 Mercury Dime DDO - FS-101
Maybe it is the angle but the missing portion does not appear... “rounded”. 🤔
You are correct, and that is actually a very good indicator that the clip is authentic. Under pressure during the strike, metal will flow between the dies and into the area of missing planchet material. Areas of lowest relief will show more movement, making the arc of the clip look wavy. Often on larger clips, you will see a small "hook" where metal flowed against the collar and into the clip. This coin has both of those hallmarks.
Sean Reynolds
"Keep in mind that most of what passes as numismatic information is no more than tested opinion at best, and marketing blather at worst. However, I try to choose my words carefully, since I know that you guys are always watching." - Joe O'Connor
Thanks @seanq, that makes perfect sense.
Is there any "Blakesly" effect?
Looks copper to me, from the photo
You guys are way overthinking this. There is a simple way to test. Just make a small "teeter-totter" and adjust one end so that a post-82 penny doesn't tip its side, but a pre-82 penny will.
I make mine with a cheap bic pen and a popsicle stick or an emory board.
the balance tool

weighing a zinc penny

weighing a copy penny

My strategy is about collecting what I intend to keep, not investing in what I plan to sell.
It is a clip. The weight is less than normal.
Interesting question. I bought a "discriminator" machine years ago that sorts coins based on metal alloy. Mainly used to sort Cu vs Zn cents. @rmorgan 's method would work too on regular cents, but the clipped cent won't work since it will weigh differently.
This would only work if I had a copper or zinc clip of the exact same size. I did weigh a few coins from my collection which seemed close but it is inexact.
Sean Reynolds
"Keep in mind that most of what passes as numismatic information is no more than tested opinion at best, and marketing blather at worst. However, I try to choose my words carefully, since I know that you guys are always watching." - Joe O'Connor
Specific Gravity is your friend!
@seanq said:
I realized after posting what the original problem was. Sorry for answering a different problem.
I do think that using the calculus as you did is the best approach. The weight difference between copper and zinc is enough that even if you are off a bit on your calculations, you should be seeing an obvious answer.
My strategy is about collecting what I intend to keep, not investing in what I plan to sell.
Find somebody that owns a metal detector...it will take about two seconds and you'll know.
A+ on your homework, extra credit for showing your work!
We actually have a low end metal detector that my son uses around the yard. How would I use it to identify the composition of the coin?
Sean Reynolds
"Keep in mind that most of what passes as numismatic information is no more than tested opinion at best, and marketing blather at worst. However, I try to choose my words carefully, since I know that you guys are always watching." - Joe O'Connor
Drill a 1/4" hole and see what comes out.
Very simple test.
Put a known copper and a known zinc on the ground and move the wand over them. There will likely be a sound difference in the audible tone.
bob
When do you expect to receive the coin? Would be interesting to compare your calculations with the actual weight.
—— But in the meantime
An experiment to try (this is non-destructive to the pictured cent):
Get one copper and one copper clad zinc. These will be destroyed in the process so pick spenders.
Trace the outline of missing portion on the two cents.
A vice would be helpful.
Cut, grind, sand, dremel or whatever is necessary to remove the portion of the two cents so it is as symmetrical as possible to the pictured cent.
Weigh and compare.
Metal detectors give very different readings for copper and zinc cents. Details of why get into aspects of metallurgy and magnetism that hurt my brain right now. Bottom like that matters is "very different".
The one caveat is that dimes/quarters/halves also give different readings on a metal detector even though they have the same composition, so size plays a role also.
Find two clips of similar size, one known to be copper and one known to be zinc. For anyone else that could be a tough ask. For you I bet you can manage :-) Compare the detector readings for the two knowns and make sure you can tell the difference. Then see which of them is a match to the unknown.
Sorry this is a little late. This is in fact a zinc coin based on the following:
1) A regular copper coin would weigh 3.1 grams. A zinc coin would weigh 2.5g (approx).
2) The clipped coin weighs 1.5g.
3) the center point of the circle rests on the existing coin, not in the clipped portion (I verified this using a simple vision measurement tool).
4) You can draw a straight line through the center of the coin and two points on the perimeter without the line touching the clipped portion. This means >50% of the coin remains. In fact, it's closer to 60-70%. If it were copper, it would weigh significantly more than 1.5g.
This is the same idea you had with the formulas, but you don't need them in this case. It is enough evidence that it is zinc.
Otherwise I would use a metal detector if you have one available, as was suggested above.
Aercus Numismatics - Certified coins for sale
So I received the coin today and confirmed the weight at 1.5g. I tried to check the specific gravity but my scale is not sensitive enough to give a definitive reading. Based on feel and sound (ok, so I dropped it on the table once or twice), I do think the coin is zinc.
This coin is signicant because it was one of just three left on my want list. Assuming this is zinc, I only need the 1982 small date zinc and the 2006 for a complete 100 year set of clips.
Sean Reynolds
"Keep in mind that most of what passes as numismatic information is no more than tested opinion at best, and marketing blather at worst. However, I try to choose my words carefully, since I know that you guys are always watching." - Joe O'Connor
Interesting "collection" challenge. Congrat's on getting down to only TWO left!
Well then... Congrats are in order.
Congrats!
Two more to go.