Options
PVC vs Acetone & Coins
 TwoSides2aCoin
Posts: 44,572 ✭✭✭✭✭
TwoSides2aCoin
Posts: 44,572 ✭✭✭✭✭
Acetone in liquid form doesn't work on coins. Polyvinyl chloride in solid form does that.
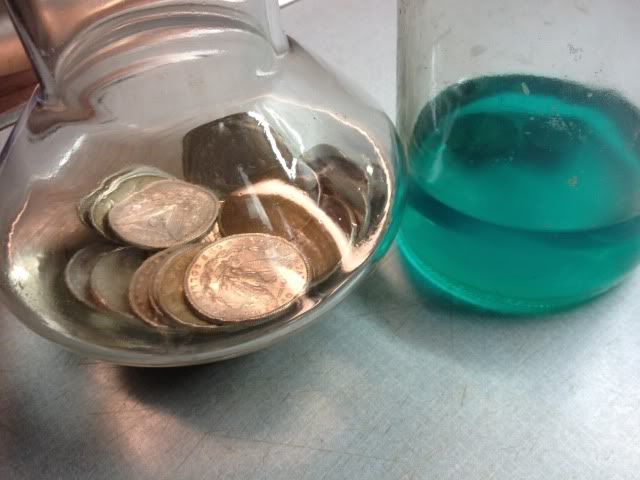
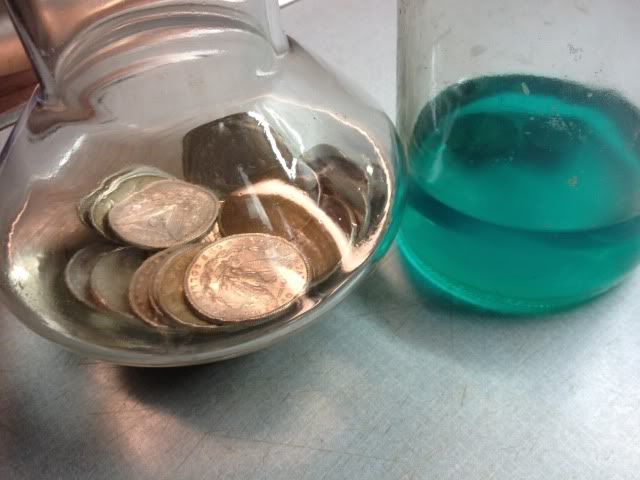
0
Comments
<< <i>What's that green liquid? It sure doesn't look like acetone. >>
It sure is acetone. Full of green goo. Filtered through coffee (paper) filters several times, it's the leftover sediment that colors the liquid.
What ends up in the filter paper is a sort of green mush. Plastic. I'm not a chemist and cannot break it down into the technical terms. I just know what it is after I used it over and over. And filtered over and over, that is the end result of coins & acetone vs. PVC ...and PVC wins. It even discolors the acetone after filtering. The coins are sitting in clear acetone, and will NOT go into the green liquid. That's just the sample to exhibit.
It's really not "fun" with coins, at all.
``https://ebay.us/m/KxolR5
You lost me. What does your statement of "acetone in liquid form doesn't work on coins" mean?
I have used acetone (pure, high quality and not the stuff you use to remove nail polish). It definitely works to remove PVC and other organic matter from coins.
As for the green goo, I would not know, since I do not reuse acetone. I use a very small glass petri dish and just enough acetone to do the job. Then several rinses with running water, and a final rinse in distilled water.
Are you reusing the acetone many times?
<< <i>What ends up in the filter paper is a sort of green mush. Plastic. I'm not a chemist and cannot break it down into the technical terms. >>
The green mush is likely oligomeric or other low molecular weight residue that isn't fine enough to filter through the paper.
<< <i>Acetone in liquid form doesn't work on coins. Polyvinyl chloride in solid form does that.
I'll have a Montecristo No. 4 with that, please!!!
<< <i>You lost me. What does your statement of "acetone in liquid form doesn't work on coins" mean?
I have used acetone (pure, high quality and not the stuff you use to remove nail polish). It definitely works to remove PVC and other organic matter from coins.
As for the green goo, I would not know, since I do not reuse acetone. I use a very small glass petri dish and just enough acetone to do the job. Then several rinses with running water, and a final rinse in distilled water.
Are you reusing the acetone many times? >>
He means work on coins in a way that the acetone doesn't affect them. Sorta like a negative thought or action that eats at you [works on you] until it drives you almost bat'sass nutz.
<< <i>What's that green liquid? It sure doesn't look like acetone. >>
It's acetone with enough dissolved copper/cuprous salt in it to color it green.
<< <i>
<< <i>What ends up in the filter paper is a sort of green mush. Plastic. I'm not a chemist and cannot break it down into the technical terms. >>
The green mush is likely oligomeric or other low molecular weight residue that isn't fine enough to filter through the paper. >>
Or copper salt/chloride that won't dissolve in the acetone.
<< <i>You lost me. What does your statement of "acetone in liquid form doesn't work on coins" mean? >>
Do not take the entire statement out of context. You have a SOLID against a solid and the liquid merely dissolves the one solid (PVC) at work, against the other solid (the coin)
<< <i>I have used acetone (pure, high quality and not the stuff you use to remove nail polish). It definitely works to remove PVC and other organic matter from coins. >>
Agreed, but it's the PVC at work and causing the work.
<< <i>As for the green goo, I would not know, since I do not reuse acetone. I use a very small glass petri dish and just enough acetone to do the job. Then several rinses with running water, and a final rinse in distilled water. >>
I am reusing the green liquid for the "first bath". The clear jar in the photograph holds the "second" bath. I also run that liquid through a paper filter when finished. There is a final rinse, not shown, but see the process ? The bath (rinse, as inferred) is just a process. The acetone is re-used after filtering.
<< <i>Are you reusing the acetone many times? >>
A couple of bath units, as seen and YES. This is why the thread title is PVC vs Acetone and Coins.
PVC works hard. Maybe see how much work it causes us and realize what I'm saying. Acetone doesn't work in liquid form on coins. It works in liquid form on PVC. Likewise, PVC works on coins in solid form and works against acetone in liquid form.
I understand what I'm thinking and saying, but maybe it's just not coming out right.
``https://ebay.us/m/KxolR5
Is PVC working on your coins ?
``https://ebay.us/m/KxolR5
I understand what I'm thinking and saying, but maybe it's just not coming out right.
No, I get it now. Geesh, you guys make me feel stupid.
Thanks for the pics and lesson.
Worry is the interest you pay on a debt you may not owe.
"Paper money eventually returns to its intrinsic value---zero."----Voltaire
"Everything you say should be true, but not everything true should be said."----Voltaire
<< <i>Why are you filtering the acetone and reusing it? Acetone isn't that expensive. Use fresh acetone for each dip and a final rinse. >>
Sort of a "greenie" here, Perry. It's not because of the economy , but the ecology that I re-use it. Just my personal preference.
``https://ebay.us/m/KxolR5
Thanks numisma. I never got a degree, just did a couple years of college. Only thing I ever got an A in was debate and music theory.
Did real well in the military field, twice. But that's getting off track, and I want to keep the focus on the coin because it's the target of my affections.
Ready, Aim, FIRE ( caution people, it's highly flammable. The solution, that is)
``https://ebay.us/m/KxolR5
<< <i>It's acetone with enough dissolved copper/cuprous salt in it to color it green. >>
Sorry guy, but that it is not. It's dissolved goo (plasticizers), clear and simple. Superficially similar in color to aqueous copper salts but totally different.
<< <i>because of ...ecology that I re-use it. Just my personal preference. >>
Great that you are being considerate of Old Mother Earth, but acetone is not really considered much of an environmental hazard. You can just pour it down your sink and follow it with 10 times the volume of water.
<< <i>Just to be accurate, polyvinyl chloride (PVC) doesn't damage coins; it's the plasticizer added to the PVC which damages coins. It evaporates out of the PVC, condenses on the coin, and then eats away at the surface if left on too long. >>
Not so. The PVC degrades to form an active chlorine species which attacks the copper in 90% silver or all copper coins. The pale green color associated with PVC damage is probably due to cuprous chloride. The plasticizer is di-nonyl or di-octyl phthalate which doesn't harm normal coins at all.
<< <i>
<< <i>It's acetone with enough dissolved copper/cuprous salt in it to color it green. >>
Sorry guy, but that it is not. It's dissolved goo (plasticizers), clear and simple. Superficially similar in color to aqueous copper salts but totally different. >>
The copper/metal would likely be the only source of color. The plasticizers and other associated compounds are colorless.
<< <i>
<< <i>Just to be accurate, polyvinyl chloride (PVC) doesn't damage coins; it's the plasticizer added to the PVC which damages coins. It evaporates out of the PVC, condenses on the coin, and then eats away at the surface if left on too long. >>
Not so. The PVC degrades to form an active chlorine species which attacks the copper in 90% silver or all copper coins. The pale green color associated with PVC damage is probably due to cuprous chloride. The plasticizer is di-nonyl or di-octyl phthalate which doesn't harm normal coins at all. >>
The primary cause is the plasticizer, a minor secondary cause is PVC breakdown. "PVC itself can degrade over long periods of time, releasing small quantities of gaseous chemicals, including hydrochloric acid, chloroethylene epoxide, and formic acid. These chemicals, in turn, can cause microscopic pitting on coins, leading to hazing on a proof surface. This outgassing occurs more if the holders are exposed to excessive humidity or sunlight. With circulated coins, these concerns are minimal."
<< <i>
<< <i>
<< <i>Just to be accurate, polyvinyl chloride (PVC) doesn't damage coins; it's the plasticizer added to the PVC which damages coins. It evaporates out of the PVC, condenses on the coin, and then eats away at the surface if left on too long. >>
Not so. The PVC degrades to form an active chlorine species which attacks the copper in 90% silver or all copper coins. The pale green color associated with PVC damage is probably due to cuprous chloride. The plasticizer is di-nonyl or di-octyl phthalate which doesn't harm normal coins at all. >>
The primary cause is the plasticizer, a minor secondary cause is PVC breakdown. "PVC itself can degrade over long periods of time, releasing small quantities of gaseous chemicals, including hydrochloric acid, chloroethylene epoxide, and formic acid. These chemicals, in turn, can cause microscopic pitting on coins, leading to hazing on a proof surface. This outgassing occurs more if the holders are exposed to excessive humidity or sunlight. With circulated coins, these concerns are minimal." >>
Well one can always get some plasticizer and put a dab on a coin and let it sit.
Just sayin...
BHNC member # 184!
http://www.busthalfaddict.com
<< <i>
<< <i>
<< <i>It's acetone with enough dissolved copper/cuprous salt in it to color it green. >>
Sorry guy, but that it is not. It's dissolved goo (plasticizers), clear and simple. Superficially similar in color to aqueous copper salts but totally different. >>
The copper/metal would likely be the only source of color. The plasticizers and other associated compounds are colorless. >>
By golly, your right! I stand corrected.
<< <i>Don't they require a background check and waiting period to purchase Acetone now?
Just sayin...
If a HazMat truck shows up or the lawmakers meet in emergency session, it could happen.
``https://ebay.us/m/KxolR5
<< <i>Acetone is a great coin cleaner.... I would not reuse it though, simply due to the building combination of 'stuff' removed from the coins... could eventually become a risk factor to metals. No idea what the chemical 'soup' really is... so many things accumulate on coins. I always rinse with fresh acetone, then alcohol, then hot running water. Distilled water rinse for proofs is recommended. Cheers, RickO >>
Does it work on Copper? I just bought a bunch of PVC Lincolns. They were beyond cheap, but the are in need of a bath??
<< <i>Does it work on Copper? >>
Yes!
Walgreens
--------T O M---------
-------------------------
I get mine at Home Depot...
Well, just Love coins, period.
<< <i>I understand what I'm thinking and saying, but maybe it's just not coming out right.
No, I get it now. Geesh, you guys make me feel stupid.
Thanks for the pics and lesson. >>
I wanted to hear the story about failing fencing (though, I probably would have joined you in the 'F'lag club), until lostincoins described his water in a bucket for a week dip/rinse method. I don't think there'll be enough time for the fencing story now. (just raggin' on ya numisma...though, if I brought home a 3.6, WHOOO, woulda been good times, that's if I had hit the books as hard as I hit offensive linemen, but, then, I just MIGHT have passed fencing!...lol)