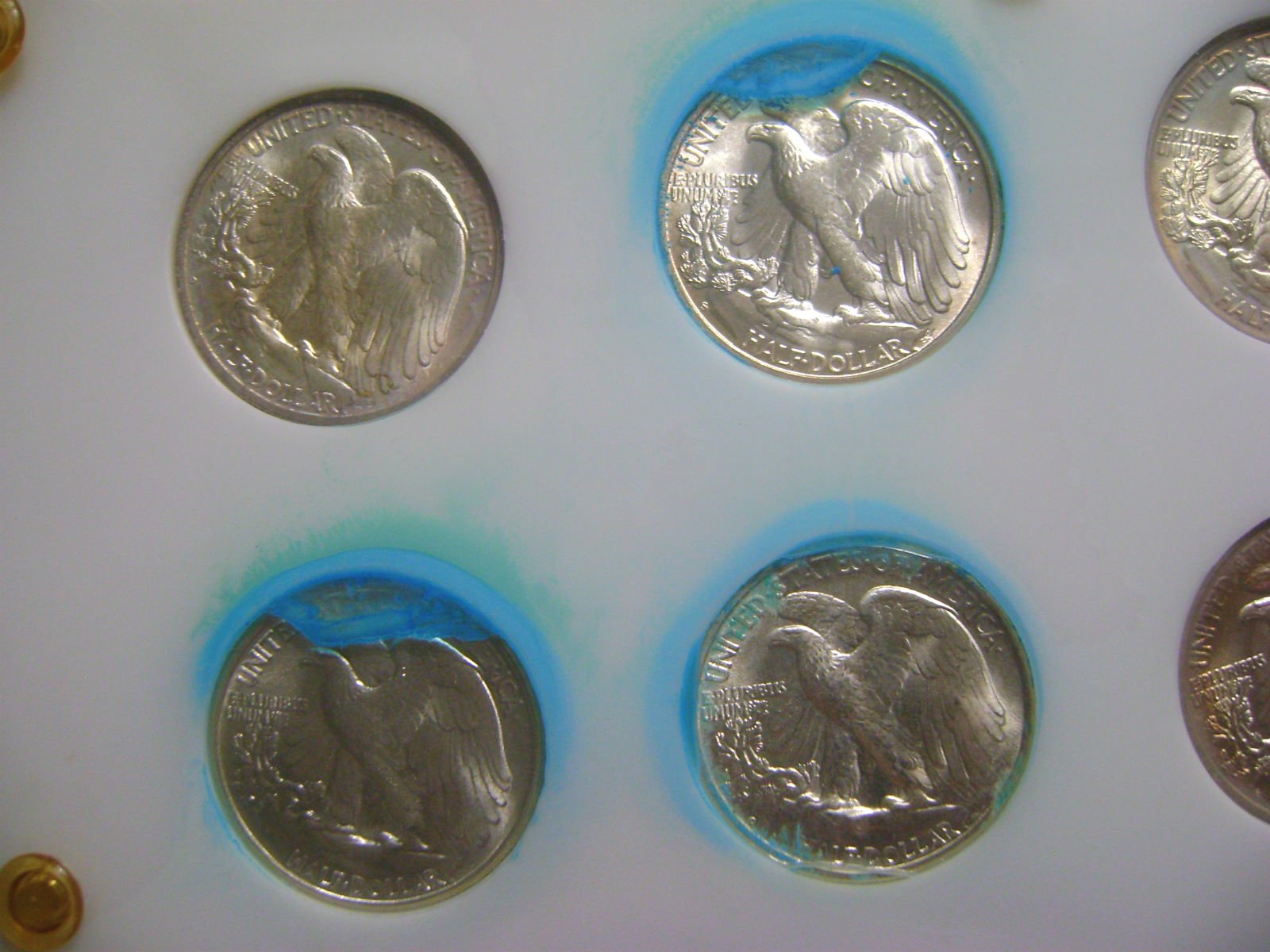
Blue stuff ruined Walkers in Capital holder - never seen anything like this
In the 1990s, I put a nice short set of Walkers together in a Capital holder. They were well-matched and brilliant, mark free, but probably wouldn't go 65. Been back and forth from safe deposit box, home safe, a few shows, for 25 years. Obviously I liked it enough to hold on to it. Hadn't looked at it in about 2 years -- it's been in a home safe with no moisture problems . I decided to bring it to the last show of the year, and wow! 4 coins all adjacent to each other covered in blue with the color spilling over onto the white plastic.
Soap and water wouldn't remove it, but an overnight soak in MS70 ( a detergent) took the blue crusty stuff off, but revealed what looks like severe PVC damage...acetone soak overnight did not remove any of this. Also not removable, some dull white areas where it looks like someone did a nitric acid test.
Photos of coins inside holder and after MS70 soaking.



Comments
Wow dude that looks crazy! Are those Capital holders made of PCV?
Capital holders don't do that. Somebody worked on those coins before putting them in the holder,and didn't rinse them properly.
Wow....... what happened to the poor walkers? She just wanted to take a walk and that happened.
Collector
Over 100 Positive BST transactions buying and selling with 57 members and counting!
instagram.com/klnumismatics
I agree. Those holders are inert as far as I have always understood it.
I suppose there is a slim chance that something on the coins reacted with the plastic, otherwise the reaction was on the coin and spread to stain the holder.
Capital holders just don't do that.
Ouch
Yikes! I don’t think capital holders will do that. Must’ve been something on the coin beforehand.
I don’t think capital holders will do that. Must’ve been something on the coin beforehand.
Sometimes, it’s better to be LUCKY than good. 🍀 🍺👍
My Full Walker Registry Set (1916-1947):
https://www.ngccoin.com/registry/competitive-sets/16292/
these Capital holders are famous for being very good for long-term storage, so something managed to get in there.
Wow ... that's really odd ... and sorry to see how it has damaged your coins.
It' hard to fathom that the coins rested safely in the Capital Plastics holder for 25 years and then 'suddenly' turned.
See http://www.doubledimes.com for a free online reference for US twenty-cent pieces
There was probably some contaminant on the holder (or perhaps on the coins themselves) at the time it was assembled. As others have said, Capital Plastics holders just don't do that.
The very selective pattern of blue crud on the holder and coins is what suggests to me that something was on the holder or was transfered from the coins at those locations.
I've seen a bottle of E-Zest spill and over a period of months the coins around it grew a blue, fungus-like substance - it looks exactly like yours.
Check out my iPhone app SlabReader!
My next comment was going to be 'same color as jewel luster'.
The "burned" areas do sorta look like what happens when you forget and leave a coin in the dip overnight.
That's along my thoughts also. If they were stable for 23 of the 25 years it seems to be the only logical explanation!
Geez oh! Talk about your contamination. Reminds me of a sci-fi movie. Hope you can rescue those WLH's. Peace Roy
BST: endeavor1967, synchr, kliao, Outhaul, Donttellthewife, U1Chicago, ajaan, mCarney1173, SurfinHi, MWallace, Sandman70gt, mustanggt, Pittstate03, Lazybones, Walkerguy21D, coinandcurrency242 , thebigeng, Collectorcoins, JimTyler, USMarine6, Elkevvo, Coll3ctor, Yorkshireman, CUKevin, ranshdow, CoinHunter4, bennybravo, Centsearcher, braddick, Windycity, ZoidMeister, mirabela, JJM, RichURich, Bullsitter, jmski52, LukeMarshall, coinsarefun, MichaelDixon, NickPatton, ProfLiz, Twobitcollector,Jesbroken oih82w8, DCW
it looks to me like a dip/fluid was spilled, the set edge was temporarily placed on it, the fluid wicked into the corner, dip did work on coins - sometime during last 5 years
Frank is an old timer, I will believe his story. Memory plays tricks on all of us, sooner or later. Several events in the past year have proven that to me!
Could it possibly have been from a used holder that at one time held improperly rinsed coins?
That's my best guess.
It sure is unfortunate though.
Those holders are three layers of rigid material. They are not necessarily air or water tight so if there was a slight, almost microscopic separation somewhere due to a slight bend in a layer or slight bulge from a coin then that could create a channel for a leaked substance to migrate into some of the holes.
Bank boxes close to the floor in the vault can get cold and damp.
Wow....that is horrible....Something was introduced to that Capitol holder... spilled, leaked onto it etc., Notable that it is on the corner....was it stored upright at some point? In some container that had or came in contact with a liquid material? Cheers, RickO
I never seen anything like that before.
This. It looks like an organic growth. It can't be a chemical reaction as the amount of material wouldn't proliferate that way.
All comments reflect the opinion of the author, even when irrefutably accurate.
I can state with a fair degree of certainty that this is not due to fungal growth. Fungi typically are saprophytes... they live on dead or decaying organic matter. Unless there was organic matter, and a noticeable amount of it, fungi will not grow. Also, very few fungi produce a blue pigment, and the conditions under which this occurs is typically in a laboratory under controlled conditions.
This looks more like a chemical reaction gone awry... residual chemicals either on the surface of the coin, or on the holder... and then a nice Lewis Acid (such as water) gets into the holder to start the oxidation...
Successful BST transactions with: SilverEagles92; Ahrensdad; Smitty; GregHansen; Lablade; Mercury10c; copperflopper; whatsup; KISHU1; scrapman1077, crispy, canadanz, smallchange, robkool, Mission16, ranshdow, ibzman350, Fallguy, Collectorcoins, SurfinxHI, jwitten, Walkerguy21D, dsessom.
I think you're right, Bill. But that sure is a lot of gunk oozing out.
Pete
I’m sorry to see this whatever it is. As others have said, capital holders don’t produce that gunk. Something got on those coins before placed in the holder.
Get them out and give em all a acetone bath ASAP.
Man, that is weird. Just curious, I realize any damage to the coins(s) is paramount, but did the blue ooze do anything to the holder, as maybe a clue to what happened?
My War Nickels https://www.pcgs.com/setregistry/nickels/jefferson-nickels-specialty-sets/jefferson-nickels-fs-basic-war-set-circulation-strikes-1942-1945/publishedset/94452
Do you remember if the 3 coins were purchased together from same seller.
The more qualities observed in a coin, the more desirable that coin becomes!
My Jefferson Nickel Collection
I didn't realize Papa Smurf was a coin collector!
Where did all those chemicals come from? There is conservation of mass, you know.
Looks far more like mold
All comments reflect the opinion of the author, even when irrefutably accurate.
I suspect Colonel Mustard. Probably in the study with a lead pipe......
But seriously, that stinks.
The chemicals were likely from a previous "dip" and not rinsed properly, or something got spilled and the chemical was introduced into the holder by capillary action and pooled around the coins. As you note, matter cannot be created or destroyed... The mass of the products must equal the mass of the reactants. In this equation, we have the mass of the chemical residue left on the coin, 90% Ag, and since Capital Plastics holders are not air tight, probably water. Note that this can be simply from high humidity. There doesn't need to be a lot of a reactant in order to get dramatic results. I'll let the Physical Chemists in the crowd figure out the chemical equation.
As far as fungus goes... this looks like no fungus I've encountered in the last 35 years of working in and around clinical microbiology... 7 years of it as a microbiologist specifically working the mycology bench. Trust me, it's not a fungus... I'd stake my PhD on it. Of course, we could simply take a sample, suspend it in 40% KOH, and use a light microscope to see if there are hyphael elements indicative of fungi...
Successful BST transactions with: SilverEagles92; Ahrensdad; Smitty; GregHansen; Lablade; Mercury10c; copperflopper; whatsup; KISHU1; scrapman1077, crispy, canadanz, smallchange, robkool, Mission16, ranshdow, ibzman350, Fallguy, Collectorcoins, SurfinxHI, jwitten, Walkerguy21D, dsessom.
I don’t know why, but it reminds me of the stuff (there is a name but it escapes me) put on the eyelid/lashes.
I am a Physical Chemist which is why I say that it doesn't look like a chemical reaction. And you have a real mass conservation problem. The silver does not appear pitted and you've had a proliferation of material. There is no foreign chemical substance on the coins capable of doing that.
You also have statistics. An organic material can spread to its neighbors like that. What is the likelihood that 3 coins had the same chemical contaminant and ended up adjacent to each other in the Capitol holder.
There are numerous blue molds, however.
https://images.app.goo.gl/TJr7QK2VnruWb22X9
All comments reflect the opinion of the author, even when irrefutably accurate.
I'm with Lance on this debate!
In every image of Penicillium that you referenced, there's a large source of organic material for the organism to metabolize... rotting fruit. What, then, is the carbon source? If there were rotting lemons in the Capital Plastics holder, I'd agree...
Successful BST transactions with: SilverEagles92; Ahrensdad; Smitty; GregHansen; Lablade; Mercury10c; copperflopper; whatsup; KISHU1; scrapman1077, crispy, canadanz, smallchange, robkool, Mission16, ranshdow, ibzman350, Fallguy, Collectorcoins, SurfinxHI, jwitten, Walkerguy21D, dsessom.
I vote thiourea dip.
Why?
It's the same colors as the crust around the lid on an old jar of ezest or jeweluster.
(agree that biologicals need food)
Liberty: Parent of Science & Industry
A friend of mine getting his pharmacy doctorate and I made a GRADING FLUID once by accident.
Had NO memory of what we dumped in the flask, but dipping a circ coin made all WEAR turn pink and the fields blue.
?????
Looks more like copper sulfate xls to me. I don't think an organic mold can etch silver. Silver-eating bacteria?
"Blue stuff" was a description of an ore (sulfide of silver) during the big Comstock lode silver bonanza.
Pacific Northwest Numismatic Association
I hope it wasn't the coin show mustard that everyone talks about!
Silver is both antibacterial and bactericidal (depending on the concentration and the organism). Colloidal silver is anti-fungal. It's hard to image that 90% silver coins would support growth of bacteria or fungi.
See http://www.doubledimes.com for a free online reference for US twenty-cent pieces
If this is dip residue and assuming no intervening event ... what would cause this to manifest a quarter century after the coins were placed in the holder? What changed from when the coins were first inserted?
See http://www.doubledimes.com for a free online reference for US twenty-cent pieces
Hmmm, Maybe his dog whizzed on it when he wasn't looking?
In my extremely limited experience, dip residue turns brown. Additionally, the "blue" is raised on the coin's surface possibly with a consistency of powder.
What does it taste like?
Very bitter blueberry - then you die.
Yikes
There is a carbon-based polymer. You have the same but opposite issue with the "chemical reaction" - what is the compound that is growing and spreading independent of anything on the coin? Note that the blue compound has "grown" into the plastic beyond where the coin is located. That is not a chemical reaction on the surface of the coin.
Without a sample, we'll never know for sure.
If the OP wants to scrape some off and send it to me, I have a complete Analytical laboratory at my disposal. Although I'd start with a microscope.
All comments reflect the opinion of the author, even when irrefutably accurate.