@Gavenadam1 said:
Unaware & un-self taught individuals usually believe everything they read or hear or think about history. I usually believe what I see or observe and can reproduce.. fundamentals of scientific study.
I guess that explains how you managed to make up brand new science.
There are NO REVERSE PROOF Morgans. The coin you show looks like some fake hobby coins that you see in "Million dollar Morgan" sets and the like. Even if it were a real, polished coin, it is NOT a "reverse proof". A reverse proof coin would have proof devices against a matte background.
Great joke, this thread. Welcome to the boards.
All comments reflect the opinion of the author, even when irrefutably accurate.
@Gavenadam1 said:
t was incredibly difficult to mine gold in this region because of "that infernal blue stuff," a rich blue clay that impeded mining efforts. Savvy miners reasoned there had to be a reason why the clay was blue, so they took samples to San Francisco for testing. The results showed that this clay contained some of the purist silver ever found.
At first, only a scant few knew about the clay. They vowed to keep it a secret. True to human nature, this did not occur. Within days, tens of thousands of miners were flocking to the area, and everyone was staking a claim.
Even Samuel Clemens, aka Mark Twain, said he staked a claim. His story is that he and three partners spent six months in a cabin drinking, playing cards, digging and panning. They found a rich vein of silver and he spent ten days dreaming of what it would be like being a multi-millionaire. At that time, work on a new claim needed to start within 10 days. During that time he was called away to visit a sick friend. He thought the partners were working at the strike, but that was not the case. In the end, someone else had staked claim to their find.
That's a great story about the COMSTOCK LODE.
You can sometimes determine the source of metal by very trace heavy metals in the refined material. You will not have any "blue clay" visible in the refined metal. The blue is a mineral not pure silver. So only X-ray analysis of the metal will yield this trace metal information.
All comments reflect the opinion of the author, even when irrefutably accurate.
I refine silver with nitric acid all the time. YouTube it. It is blue & not colorless. Don’t try it if you’re immature or don’t know what you’re doing though.....
@Gavenadam1 said:
I refine silver with nitric acid all the time. YouTube it. It is blue & not colorless. Don’t try it if you’re immature or don’t know what you’re doing though.....
I have a Ph.D. in Chemistry and 30 years experience.
You can use nitric acid or any oxidizing acid to dissolve silver salts. The blue color is NOT FROM SILVER but something else in the unrefined silver. Silver salts, like silver nitrate, are colorless. Nitric acid has a yellowish hue. If you dissolve pure silver in nitric acid, it will be slightly yellow from the nitric acid, the silver ion is COLORLESS.
When you are "refining" silver, you have impurities that you are trying to separate. That is likely where the blue color is coming from, probably copper as copper nitrate is blue in solution.
Here's a picture of a beaker of silver nitrate and silver nitrate with copper in it. Notice the total lack of color before reaction. Notice how it turns blue after the copper oxidizes and dissolves:
All comments reflect the opinion of the author, even when irrefutably accurate.
@Gavenadam1 said:
I refine silver with nitric acid all the time. YouTube it. It is blue & not colorless. Don’t try it if you’re immature or don’t know what you’re doing though.....
This is Jessup, isn't it?
All comments reflect the opinion of the author, even when irrefutably accurate.
@Gavenadam1 said:
Not X-ray but like it with a laser spectral device. You can even tell the origin of most metals.
Again, a little knowledge is...well, whatever...
Visible lasers are next to useless for metal identification. Metals don't absorb the light, they reflect it. The metals, specifically the electrons in the metals, do absorb X-rays. That is why X-ray fluorescence and related techniques are used for metal identification.
You can use spectral imagers for MINERALS in their crystalline form. But if you have any coins struck on quartz planchets, please show us pictures.
X-ray data can tell you WHAT the metal is and what FORM the metal is in (pure metal, bonded to oxygen, etc.). You can NOT tell "origin" other than by implication of the composition.
Adding a spectral imager to the mix will allow you to visualize the object based on the reflectivity of different wavelengths.
All comments reflect the opinion of the author, even when irrefutably accurate.
@Gavenadam1 said:
3 questions.
1. What number on the periodic table is it on?
2. What color does it turn when mixed with acid in a laboratory to purify it?
3. What medal can be turned int Silver with acid?
@ElmerFusterpuck said:
I still like turtles. As far as I know, no coins have been minted using actual turtle shells.
Oh, and 42.
Most sensible comment in the thread. 42 is indeed the answer to everything.
Actually there are some good comments in this thread from people (not the OP of course) that know a thing or two about numismatics, history, and metallurgy.
Love the criticism & any useful info. Ty. I know who I am thus not ever a conformist to any type of human cliche etc. I took several chemistry classes & did use all safety and created by missing junk silver in nitric acid —-blue “Silver Nitrate” you put the element under it in the silver nitrate & the reaction bumps it up to .999 silver which in this case is copper. The reaction creates a mustard gas so yes, equipment & maturity needed. The particles left from the copper is pure silver. & the leftover solution quick silver.
I wonder about the blue sand also and still am researching as to how that would’ve happened. God Bless you all! I’ll keep everyone informed on that coin & it already is sent btw... I’ll also try to post some of those blue hued early Morgans.
@Insider2 said: @jmlanzaf said: "... You can NOT tell "origin" other than by implication of the composition.
Will you simplify and expand on this for all of us? Thanks.
Sure. Silver is silver is silver. [We'll ignore isotopes, but they all behave identically also.] A silver atom, no matter where it was mined, no matter how it was refined or treated, will behave exactly the same in any scientific test you put it in.
If you have a bar of 100.000 00000% pure silver, all the silver in it behaves identically. It could come from all over the world and you couldn't tell where any of it came from. Silver is silver is silver.
But there is no such thing as 100.0000000% pure anything. Silver, even a chunk of silver pulled right out of a "pure" silver vein has other chemical species in it, including trace metals. And by trace, I mean parts per million or slightly more. Different geological formation have different mixes of these trace elements. So, it MAY be possible to identify a sample of silver as coming from the Comstock Lode, for example, if Comstock silver was known to have 10 ppm Uranium and 100 ppm Iridium.
This is not always possible to do, especially once samples from different sources are mixed. Or if different refining processes were used that changed the trace metal composition. But it has been useful for things like pioneer gold where it came from a specific region and was refined and assayed on site or nearby and so there are samples to compare to.
It would be even more useful to geologists with unrefined ore samples, but that would be less useful to numismatists.
All comments reflect the opinion of the author, even when irrefutably accurate.
@Gavenadam1 said:
Love the criticism & any useful info. Ty. I know who I am thus not ever a conformist to any type of human cliche etc. I took several chemistry classes & did use all safety and created by missing junk silver in nitric acid —-blue “Silver Nitrate” you put the element under it in the silver nitrate & the reaction bumps it up to .999 silver which in this case is copper. The reaction creates a mustard gas so yes, equipment & maturity needed. The particles left from the copper is pure silver. & the leftover solution quick silver.
I wonder about the blue sand also and still am researching as to how that would’ve happened. God Bless you all! I’ll keep everyone informed on that coin & it already is sent btw... I’ll also try to post some of those blue hued early Morgans.
AS I SAID: the copper is blue, silver is not.
It may also be copper minerals that make the "blue clay" blue. Most silver compounds I'm familiar with range from pale yellow to white to light grey. It is not a very colorful element on its own.
All comments reflect the opinion of the author, even when irrefutably accurate.
@MFeld said:
This looks to be the same poster who has started and/or posted to a number of threads about (alleged) O-mint Proof Morgan dollars on another forum. In each case, the posted pictures were of obvious business strikes - one or more of them, polished.
@jmlanzaf said: "Different geological formation have different mixes of these trace elements.""
That's what I wanted you (as a chemist) to tell everyone. Numismatists in several fields have published analysis of coins and traced where their metal was mined. Many counterfeits have been identified because of their composition including the trace elements that should not be in the coins.
@JBK said:
But the OP's Morgan is really shiny - it MUST be a proof!
I'm told it is a BRANCH MINT REVERSE PROOF, therefore unique.
That would also probably make it the first reverse proof ever struck. Does anyone know what the first reverse proof coin issued was? I'm not aware of anything in the 19th century.
All comments reflect the opinion of the author, even when irrefutably accurate.
@JBK said:
But the OP's Morgan is really shiny - it MUST be a proof!
I'm told it is a BRANCH MINT REVERSE PROOF, therefore unique.
That would also probably make it the first reverse proof ever struck. Does anyone know what the first reverse proof coin issued was? I'm not aware of anything in the 19th century.
I believe there's a small chance that the OP is in possession of a legitimate branch mint proof. I see no evidence of cleaning or harsh polishing. If I were him, I would definitely submit the coin to our host for professional feedback. It's well worth the cost for a potential rarity like this.
@Gavenadam1 said:
Still love you guys, was trying to joke & as a missionary/Christian or believer in Christ, I really mean it. I thank you all for your help.
First of all, good service working in the "field"! (check my sig line). Also sorry for your loss. Your grandmother probably was able to have many old stories to tell you, being 90? So thoughtful of leaving you such gems. Those coins you posted are marvelous! Oh yes, Welcome to school. I say school because you'll be taught in many areas. Such as in ethics, manners and in business. I'm sure you learned a few things just by this thread. Never be a stranger and post often. Ask any questions you may and the most important thing, have FUN! -joey
"Jesus died for you and for me, Thank you,Jesus"!!!
--- If it should happen I die and leave this world and you want to remember me. Please only remember my opening Sig Line.
Comments
I guess that explains how you managed to make up brand new science.
There are NO REVERSE PROOF Morgans. The coin you show looks like some fake hobby coins that you see in "Million dollar Morgan" sets and the like. Even if it were a real, polished coin, it is NOT a "reverse proof". A reverse proof coin would have proof devices against a matte background.
Great joke, this thread. Welcome to the boards.
All comments reflect the opinion of the author, even when irrefutably accurate.
That's a great story about the COMSTOCK LODE.
You can sometimes determine the source of metal by very trace heavy metals in the refined material. You will not have any "blue clay" visible in the refined metal. The blue is a mineral not pure silver. So only X-ray analysis of the metal will yield this trace metal information.
All comments reflect the opinion of the author, even when irrefutably accurate.
I refine silver with nitric acid all the time. YouTube it. It is blue & not colorless. Don’t try it if you’re immature or don’t know what you’re doing though.....
Not X-ray but like it with a laser spectral device. You can even tell the origin of most metals.
I would enjoy a "Leave it to Beaver" rerun, so why not? I'm not givin' a dam -yet being entertained!
@Gavenadam1 "I refine silver with nitric acid all the time."
This could be part of the problem.... you have failed to do it in a well ventilated area and now you are having a bad acid trip.
I have a Ph.D. in Chemistry and 30 years experience.
You can use nitric acid or any oxidizing acid to dissolve silver salts. The blue color is NOT FROM SILVER but something else in the unrefined silver. Silver salts, like silver nitrate, are colorless. Nitric acid has a yellowish hue. If you dissolve pure silver in nitric acid, it will be slightly yellow from the nitric acid, the silver ion is COLORLESS.
When you are "refining" silver, you have impurities that you are trying to separate. That is likely where the blue color is coming from, probably copper as copper nitrate is blue in solution.
Here's a picture of a beaker of silver nitrate and silver nitrate with copper in it. Notice the total lack of color before reaction. Notice how it turns blue after the copper oxidizes and dissolves:

All comments reflect the opinion of the author, even when irrefutably accurate.
If a little knowledge is a dangerous thing...well....
All comments reflect the opinion of the author, even when irrefutably accurate.
This is Jessup, isn't it?
All comments reflect the opinion of the author, even when irrefutably accurate.
Again, a little knowledge is...well, whatever...
Visible lasers are next to useless for metal identification. Metals don't absorb the light, they reflect it. The metals, specifically the electrons in the metals, do absorb X-rays. That is why X-ray fluorescence and related techniques are used for metal identification.
You can use spectral imagers for MINERALS in their crystalline form. But if you have any coins struck on quartz planchets, please show us pictures.
X-ray data can tell you WHAT the metal is and what FORM the metal is in (pure metal, bonded to oxygen, etc.). You can NOT tell "origin" other than by implication of the composition.
Adding a spectral imager to the mix will allow you to visualize the object based on the reflectivity of different wavelengths.
All comments reflect the opinion of the author, even when irrefutably accurate.
Heather, I have been triggered by this thread.
SAFE SPACE NEEDED !!
Please refund the 5 minutes of my life lost in reading STUPID !!
Yes, yes, and sometimes.
Most sensible comment in the thread. 42 is indeed the answer to everything.
Actually there are some good comments in this thread from people (not the OP of course) that know a thing or two about numismatics, history, and metallurgy.
But the effort is wasted on the troll.
@jmlanzaf said: "... You can NOT tell "origin" other than by implication of the composition.
Will you simplify and expand on this for all of us? Thanks.
I wonder when this thread will be closed.
I think the OP should send his Branch Mint Proof to PCGS for certification.
Indeed it is...42 is the Answer to the Ultimate Question of Life, The Universe, and Everything. Just ask Deep Thought.
Lance.
Love the criticism & any useful info. Ty. I know who I am thus not ever a conformist to any type of human cliche etc. I took several chemistry classes & did use all safety and created by missing junk silver in nitric acid —-blue “Silver Nitrate” you put the element under it in the silver nitrate & the reaction bumps it up to .999 silver which in this case is copper. The reaction creates a mustard gas so yes, equipment & maturity needed. The particles left from the copper is pure silver. & the leftover solution quick silver.
I wonder about the blue sand also and still am researching as to how that would’ve happened. God Bless you all! I’ll keep everyone informed on that coin & it already is sent btw... I’ll also try to post some of those blue hued early Morgans.
Make sure you position the coin in the light just so (tip it) in order to make its polished surface look "blue" as this one does:
I’m posting a few of mine for those interested
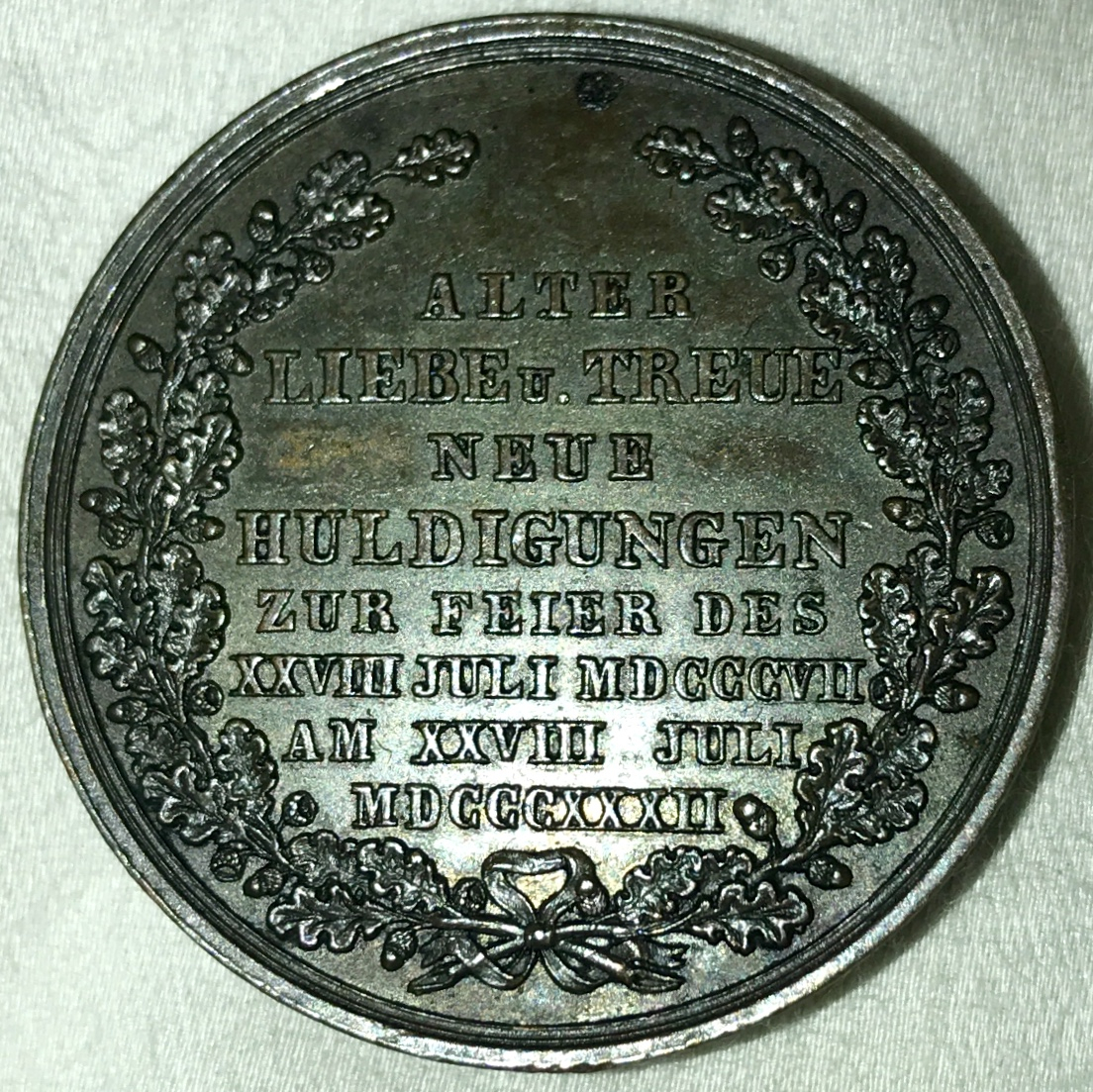

Now, that's a nice piece of jewelry. Was it Grandma's?
It's got to be a troll, and a pretty entertaining one.
If not, I can sell you a bunch of polished Morgans. I mean, super rare, blue clay silver, reverse proofs.
Sure. Silver is silver is silver. [We'll ignore isotopes, but they all behave identically also.] A silver atom, no matter where it was mined, no matter how it was refined or treated, will behave exactly the same in any scientific test you put it in.
If you have a bar of 100.000 00000% pure silver, all the silver in it behaves identically. It could come from all over the world and you couldn't tell where any of it came from. Silver is silver is silver.
But there is no such thing as 100.0000000% pure anything. Silver, even a chunk of silver pulled right out of a "pure" silver vein has other chemical species in it, including trace metals. And by trace, I mean parts per million or slightly more. Different geological formation have different mixes of these trace elements. So, it MAY be possible to identify a sample of silver as coming from the Comstock Lode, for example, if Comstock silver was known to have 10 ppm Uranium and 100 ppm Iridium.
This is not always possible to do, especially once samples from different sources are mixed. Or if different refining processes were used that changed the trace metal composition. But it has been useful for things like pioneer gold where it came from a specific region and was refined and assayed on site or nearby and so there are samples to compare to.
It would be even more useful to geologists with unrefined ore samples, but that would be less useful to numismatists.
All comments reflect the opinion of the author, even when irrefutably accurate.
I appreciate jmlanzaf trying. There's some borderline delusional stuff going on with the OP.
IG: DeCourcyCoinsEbay: neilrobertson
"Numismatic categorizations, if left unconstrained, will increase spontaneously over time." -me
AS I SAID: the copper is blue, silver is not.
It may also be copper minerals that make the "blue clay" blue. Most silver compounds I'm familiar with range from pale yellow to white to light grey. It is not a very colorful element on its own.
All comments reflect the opinion of the author, even when irrefutably accurate.
shh...Rick probably already bought movie tickets at the pre-release discount
All comments reflect the opinion of the author, even when irrefutably accurate.
Oh, THAT's where Conder101 wound up!! I miss his input on this forum. I'm glad this thread wasn't a total loss!
Successful BST transactions with: SilverEagles92; Ahrensdad; Smitty; GregHansen; Lablade; Mercury10c; copperflopper; whatsup; KISHU1; scrapman1077, crispy, canadanz, smallchange, robkool, Mission16, ranshdow, ibzman350, Fallguy, Collectorcoins, SurfinxHI, jwitten, Walkerguy21D, dsessom.
@jmlanzaf said: "Different geological formation have different mixes of these trace elements.""
That's what I wanted you (as a chemist) to tell everyone. Numismatists in several fields have published analysis of coins and traced where their metal was mined. Many counterfeits have been identified because of their composition including the trace elements that should not be in the coins.
But the OP's Morgan is really shiny - it MUST be a proof!
Did I just see a “PCCB” slab?
If so, then homemade slabber.
I'm told it is a BRANCH MINT REVERSE PROOF, therefore unique.
That would also probably make it the first reverse proof ever struck. Does anyone know what the first reverse proof coin issued was? I'm not aware of anything in the 19th century.
All comments reflect the opinion of the author, even when irrefutably accurate.
I believe there's a small chance that the OP is in possession of a legitimate branch mint proof. I see no evidence of cleaning or harsh polishing. If I were him, I would definitely submit the coin to our host for professional feedback. It's well worth the cost for a potential rarity like this.
First of all, good service working in the "field"! (check my sig line). Also sorry for your loss. Your grandmother probably was able to have many old stories to tell you, being 90? So thoughtful of leaving you such gems. Those coins you posted are marvelous! Oh yes, Welcome to school. I say school because you'll be taught in many areas. Such as in ethics, manners and in business. I'm sure you learned a few things just by this thread. Never be a stranger and post often. Ask any questions you may and the most important thing, have FUN! -joey

"Jesus died for you and for me, Thank you,Jesus"!!!
--- If it should happen I die and leave this world and you want to remember me. Please only remember my opening Sig Line.Dude, I just noticed how many views you already have in just three days. Almost 2000?
"Jesus died for you and for me, Thank you,Jesus"!!!
--- If it should happen I die and leave this world and you want to remember me. Please only remember my opening Sig Line.Train wrecks always get viewers.
All comments reflect the opinion of the author, even when irrefutably accurate.
For the haters...

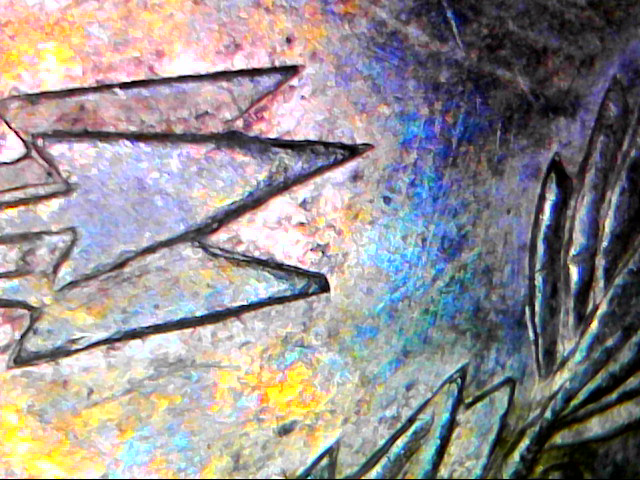



I even went microscopic to show the color...
I'm checking my collection for reverse branch mint proofs right now. Have found two so far...
Smitten with DBLCs.
You did a perfect job - just as I asked. The blue color on that polished coin is easy to see.
Comedy gold...