The Coloration Of Charlotte and Dahlonega Gold
 FlyingAl
Posts: 4,123 ✭✭✭✭✭
FlyingAl
Posts: 4,123 ✭✭✭✭✭
I must preface this post with stating that a lot of the work to understand this coins has already been done, so I must give credit where credit is due. Most of this research is paraphrased from an excellent article on these coins by Carl Lester, linked here. I HIGHLY RECOMMEND anyone who is interested reads it, as it explains the topic better than I ever could.
Link: https://www.goldrushgallery.com/news/coloration.html
I've also pulled info from various sources from the old forums, mostly from @CharlotteDude for the gold delivery figures shown later on. One last note - this is a quick post, and is far from comprehensive.
.
.
.
What Are We Talking About?
Many advanced numismatists have noticed over the years, early Dahlonega and Charlotte gold possesses a unique color, often described as a "greenish gold". The color ranges from barely noticeable to quite noticeable, and is shown quite well by the following examples:
Barely noticeable:
.
.
Very noticeable:

.
.
Philadelphia example:
.
.
.
.
Why The Color?
The alloy of US Mint gold coinage was limited by law to be 90% pure gold, and ten percent alloy. That alloy was allowed to contain up to 5% silver, with the balance being copper. Essentially, copper had to be at least 5%. The more copper, the redder the color, and the stronger the color. The more silver, the lighter and "greener" the color.
The US Mint shifted its preferred alloys slightly in the 1830s and 1840s, phasing out the silver when it was possible. Source gold from the Georgia gold rush was incredibly pure, averaging at about 95% pure. Eastern gold as a whole followed this pattern, with alloys generally containing silver as the balance. If a sample of gold came into the mint as 95% gold, 5% silver, then the addition of copper to hit a 90/5/5 ratio would make the gold coinable. Branch mints had limited capability to remove alloy that was unwanted, which is why the inclusion of silver and copper in the alloy was necessary - the inclusion of borax flux in the melting process would cause impurities to rise to the surface of the metal, where it could be skimmed. The resulting liquid generally contained gold, silver, and copper. Without specialized refining, the silver would not be removed, and thus the higher the ratio of silver in the natural ore, the higher the ratio of silver in the finished coinage.
Lester's article shows XRF analysis figures on dates for Dahlonega coinage, and we see that the coins contained silver in the following percentages:
1838-D half eagle: 4.8 % (48 parts per thousand)
1845-D quarter eagle: 5.0 % (50 ppt)
1846-D quarter eagle: 4.3 % (43 ppt)
1847-D quarter eagle: 4.3 % (43 ppt)
1847-D half eagle: 4.0 % (40 ppt)
1850-D gold dollar: 3.7 % (37 ppt)
1853-D gold dollar: 3.9 % (39 ppt)
1853-D half eagle: 3.6 % (36 ppt)
1854-D half eagle: 1.3 % (13 ppt)
1854-D gold dollar: 1.4 % (14 ppt)
1854-D half eagle: 1.3 % (13 ppt)
1855-P gold dollar: 1.13 % (11.3 ppt)
Essentially, up until about 1854, the coins contained near the maximum amount of silver, likely from the high quality of natural silver from the source gold. The question now arises - why are the late 1850s coins much lower in silver content? The answer is found in the source gold. Starting in around 1850, some of the gold used by Charlotte and Dahlonega was from California. We can see this in the following delivery records:
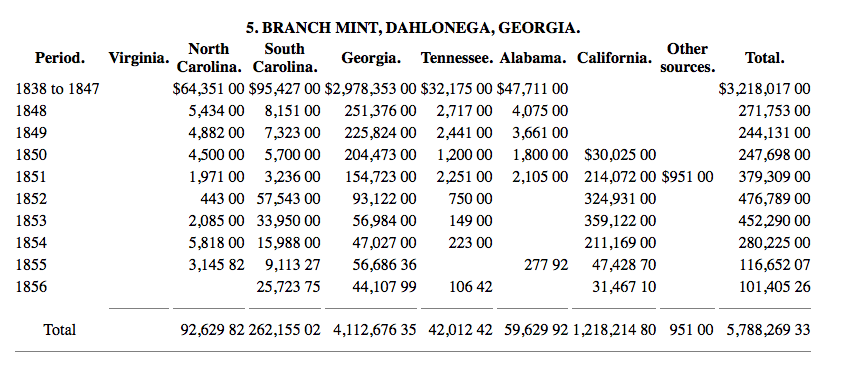
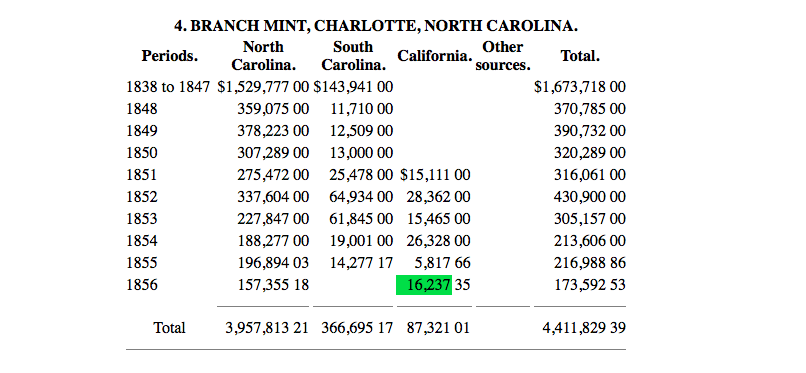
.
.
Western gold caused problems, as it had much higher rates of impurities and contained elements that could not be removed by the addition of flux. A post by @CaptHenway, who posted some information from another source on the topic, explains this well.
"When quantities of California gold began returning east, either to Georgia and the Carolinas with miners, or shipped by aggregators to New York and Boston, problems arose that required more robust refining and bullion processing. The nitric acid process was adopted at U.S. Mints and greatly expanded after 1848. Much California placer gold had a higher silver content than eastern gold, and it also contained platinum, iridium and osmium. These last three mimicked gold weight, but were worth much less. This reduced the true pure gold content and caused extensive complaint in London and elsewhere about being “short” of true gold content. California gold depositors also felt they were being treated unfairly because they were paid only for gold and the Mints kept the silver. This is when a much more robust and chemically active approach to gold and silver refining was firmly adopted."
The need for additional specialized refining would have ultimately reduced the silver content in the gold, and therefore changed the color of said coins. This explains the significant drop in silver percentage in the 1850s coinage, and the appearance change of said coinage.
Please feel free to post any Charlotte or Dahlonega gold!
Comments
Really good write up!
What else is in the XRFs? Any Iron?
Here are mine.


Proud follower of Christ!
Not sure, I didn't do them. Those were just the figures Lester's article reported.
So if you want a true type coin you want one before 1854.
Excellent write up, so my Dahlonega must have a relatively high amount of silver in it


But my Bechtler piece must have a high amount of copper in it
Mr_Spud
Great writeup !!!
XF45 pop 20 & 88 higher
Top 10 Cal Fractional Type Set
successful BST with Ankurj, BigAl, Bullsitter, CommemKing, DCW(7), Downtown1974, Elmerfusterpuck, Joelewis, Mach1ne, Minuteman810430, Modcrewman, Nankraut, Nederveit2, Philographer(5), Proofcollection, Realgator, Silverpop, SurfinxHI, TomB and Yorkshireman(3)
.
Thanks for the info!
As far as the legislation is concerned, it comes from the Coinage Act of 1792:
"SEC. 12. And be it further enacted, That the standard for all gold coins of the United States shall be eleven parts fine to one part alloy; and accordingly that eleven parts in twelve of the entire weight of each of the said coins shall consist of pure gold, and the remaining one twelfth part of alloy; and the said alloy shall be composed of silver and copper, in such proportions not exceeding one half silver as shall be found convenient; to be regulated by the director of the mint, the United States, until further provision shall be made by law. And to the end that the necessary information may be had in order to the making of such further provision, it shall be the duty of the director of the mint at the expiration of a year after commencing the operations of the said mint, to report to Congress the practice thereof during the said year, touching the composition of the alloy of the said gold coins, the reasons for such practice, and the experiment and observation which shall have been concerning the effects of different proportions of silver and copper in the said alloy."
As far as the natural composition of gold goes, I had some trouble finding reliable sources on it. The best I found was actually the link you posted, saying "The Australian gold averages 94 per cent. pure, while the California gold is only 88 percent. The Georgia gold runs from 75 to 98 per cent. pure, a general average being some- what higher than the California gold." It also lists silver as the chief impurity for two specific mines. Zinc and cadmium were not mentioned in relation to the eastern US gold.
Wasent there a complete set of these in a capital holder come threw here as well a while back? No luck here find it
I would send some credit in the direction of Doug Winter for educating those paying attention to such things:
https://raregoldcoins.com/
DOG acolyte
The Act of January 18, 1837 raised the level of fineness in gold coins from 89.9225% to 90%. It also stipulated that the 10% alloy of copper and silver could not exceed 5% in silver. At the time, the Philadelphia MInt followed a policy of using 2:1 copper to silver ratio, but in 1837 changed to a 3:1 ratio. Personnel at the mint and in Washington were influenced by the beautiful color of the French Indemnity gold received in the U.S. during 1836.
As already mentioned, the high purity and high content of silver of southern gold allowed the branch mints to minimize or eliminate the need to wash and part the gold and silver, which during their early years had limited capability to do so.
Excellent info...bookmarked!
http://macrocoins.com
Great info - here are my examples:





“In matters of style, swim with the current; in matters of principle, stand like a rock." - Thomas Jefferson
My digital cameo album 1950-64 Cameos - take a look!
Happened to be tiptoeing through the 1877 Mint Report and found these reports on the assayed gold finenesses, and silver contents, of gold coins from P, S and CC from July, 1875 to June, 1876. The silver contents are presumably parts per thousand, and consistently less than 10, which would be one percent. That was the recommended upper limit given in the Coinage Act of 1873, which may be why they bothered to do this report in 1877.
Despite its (presumably) superior technological prowess, Philadelphia tends to run just slightly higher than the western mints. I would hazard a guess that this was because the western mints tended to get consistent local gold, while Philadelphia had to process a wide variety of foreign gold coins and bars coming in through the New York Assay Office.
This is the only Dahlonega mint coin I still own. The lone P45+ and has a sticker.

.
Successful BST with drddm, BustDMs, Pnies20, lkeigwin, pursuitofliberty, Bullsitter, felinfoel, SPalladino
$5 Type Set https://www.pcgs.com/setregistry/u-s-coins/type-sets/half-eagle-type-set-circulation-strikes-1795-1929/album/344192
CBH Set https://www.pcgs.com/setregistry/everyman-collections/everyman-half-dollars/everyman-capped-bust-half-dollars-1807-1839/album/345572
Splendid write up! I’m very fond of the color on gold, I have three examples, Charlotte, Dahlonega, and Philadelphia:
My YouTube Channel
A couple of general questions about XRF analysis:
Do the XRF figures reflect the entire coin or just a small area of it?
If just a small area is tested, would that reliably represent the composition of the entire coin or would multiple XRFs of the same coin be preferable?
If the TPGs offered XRF analysis, I'd be curious how my coins did but not sure I'd pay extra and I'd hate to see a coin with something like 87% gold.
When I was at ANACS we had access to a Scanning Electron Microscope on the campus of the Colorado College for a fee. We did not use it often because of the fee, which management begrudged us paying.
Out of curiosity, one time while we had a circulated coin on the 'scope I had the operator take a test of an open area of the field, a worn but basically clean high spot, and the dark, toned area where the field met the head, which probably had some circulation schmutz in it. The field and high spot were essentially the same, while the dark angle showed all sorts of trace elements, I assume from the schmutz.
The operator helpfully suggested that for a true reading of a sample you should polish an area down to bare metal and test that. I calmly told him that this was not an option on numismatic items.
1838 Eagles. First year minted since 1803. Supposedly made from melted sovereigns and would hold a light green coloration if not messed with.
@FlyingAl I wonder if this is similar? @MrEureka ?
My 1866 Philly Mint Set
Nice compilation of info that includes my favorite branch mint. Here are a few examples representing decades of green…








P58/CAC
Half eagles of 1850 C are especially pronounced IMO. So is 1857 C.
Proud follower of Christ!
I haven’t heard of the story before, but the MS example on CoinFacts does seem to have a greenish orange look to it. Very well could be from a higher silver content.
I couldn’t find the exact info on composition for British sovereigns, but a preliminary search reveals around 2-3% silver.
Wow, really beautiful coins. That 38-C is outstanding.
A C and a D.


https://www.pcgs.com/setregistry/gold/liberty-head-2-1-gold-major-sets/liberty-head-2-1-gold-basic-set-circulation-strikes-1840-1907-cac/alltimeset/268163
Also several years of NOLA DEs are known to have ‘green’ gold when found with good originality. I assume this is the case for the other denominations as well.
>
Here’s one example.
@HillbillyCollector
Excellent point! @PeakRarities mentioned the same for some Philadelphia issues.
This post alludes to the reasoning for that - namely the origin of the gold. If it came from high silver sources (namely eastern gold) it would tend to appear lighter in color (and occasionally show a green tinge).
I was able to access Roger Burdette's From Mine To Mint, and he states that N. Carolina gold averaged .844 fine, S. Carolina at .925, Virginia and Maryland at .920, and Georgia at .950.
.
1849 may be a bit late for the gold we’re discussing. I’d need to do more research on this.
.
I think the nitric process was relatively new in 1847, but I’m not entirely sure. This may also explain the drop in silver composition we see in later years.
@CaptHenway do you know when the nitric process was commonly accepted and used?
No idea on the chemistry processes.
.
Good info, and I can get behind this theory.
If true, how would you explain the extra silver in the branch mint gold? Poor refining or some other cause?
.
Very interesting and intriguing additional info on ore processing here. Worthy of a few follow-on examples for reference.
I'll look into it when I get a chance. For now, I'm not going to entirely rule out extra silver in the native gold. Hopefully this new info can lead to a better theory or uncovers a little more. Thanks for posting it!
I think extra XRF data would be very helpful as well.