Two super-awesome toned lincolns and a Sac... including a neon Steelie!!!
I photographed these for RedBimmer (he's going to break the silence and start posting all the time soon  )... they are both very lustrous and awesomely toned pieces--the steelie being the coolest I've ever seen. I won't do a guess the grade, because I was too lazy to change the file names
)... they are both very lustrous and awesomely toned pieces--the steelie being the coolest I've ever seen. I won't do a guess the grade, because I was too lazy to change the file names 
1943 NGC MS67

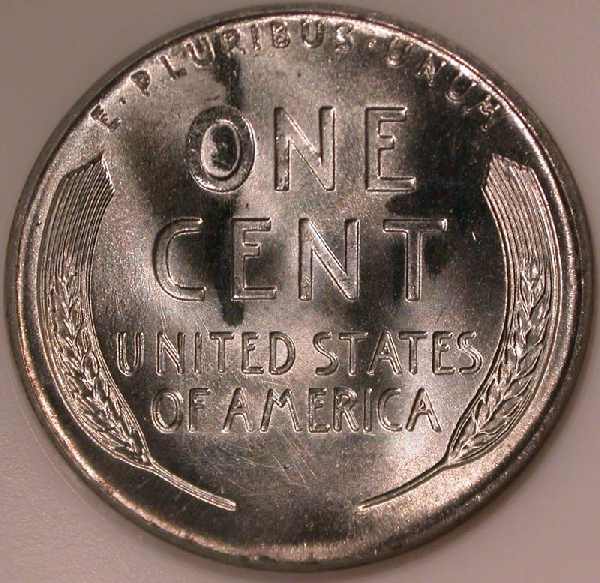
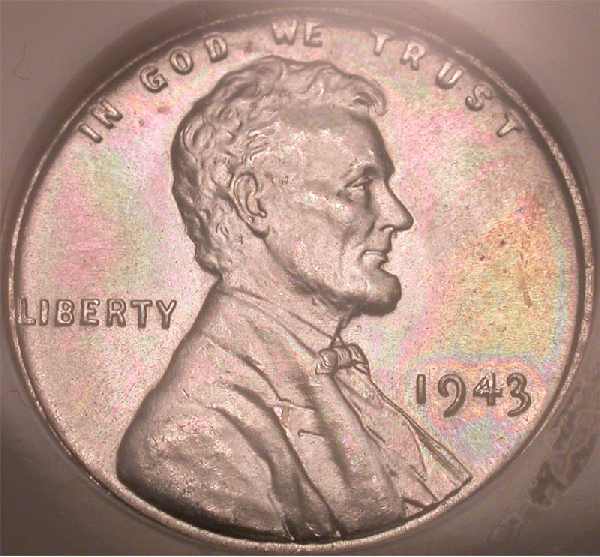

1956-D NGC MS66*

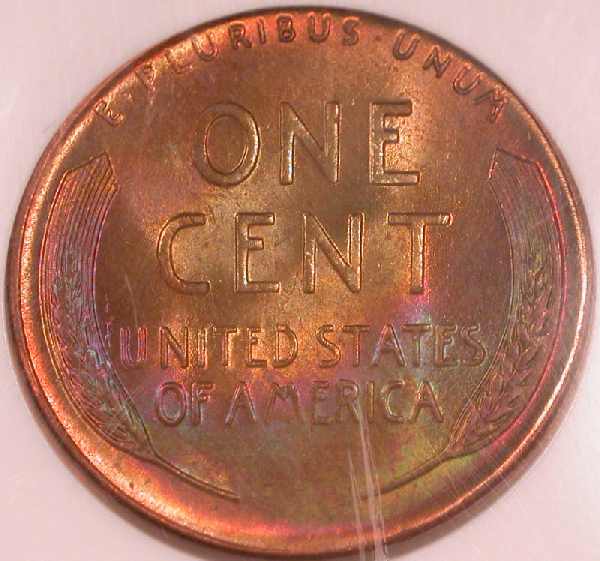

NGC MS65



Enjoy!!!
Jeremy
1943 NGC MS67

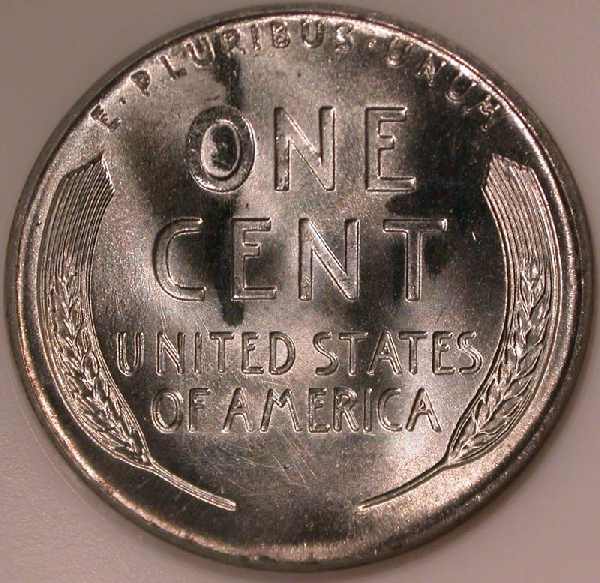
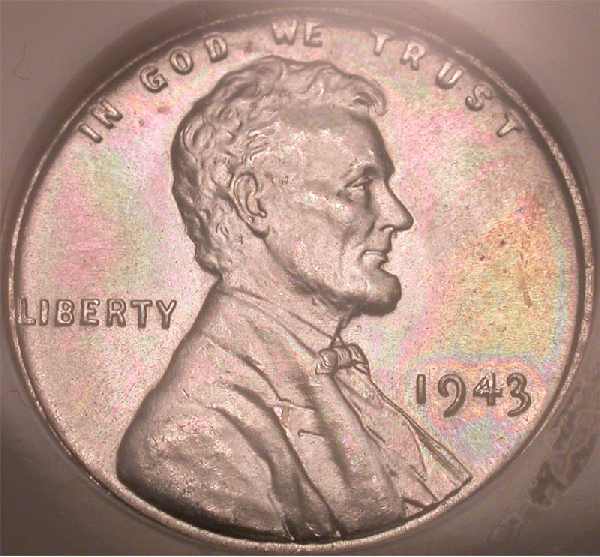

1956-D NGC MS66*

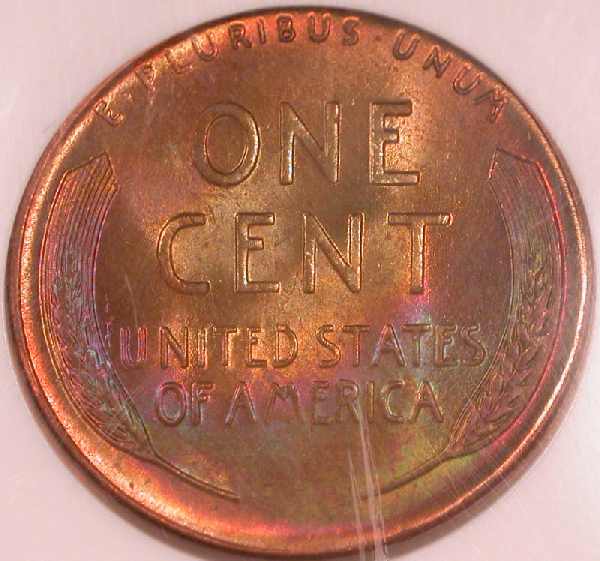

NGC MS65



Enjoy!!!
Jeremy
JK Coin Photography - eBay Consignments | High Quality Photos | LOW Prices | 20% of Consignment Proceeds Go to Pancreatic Cancer Research
0
Comments
I'm interested in hearing what people's opinions on the 1943 might be. Has anyone ever seen these with toning like that? AT? NT?
Thank You to all who care to respond!
Rob
Dennis
Like VOC Numismatics on facebook
Capped Bust Half Series
Capped Bust Half Dime Series
42/92
<< <i>Beautiful Pics!! That steelie ain't a 67 though. It's a 66. >>
Trust me... I know a number when I see it
SUPER COOL COINS, JEREMY!
Bob
PS: thanks for not including the full-blown images. You might have shut down the CU forum bandwidth!
PPS: can zinc tone? I thought it would only oxidize. Chemistry folks?
Vietnam Vet 1968-1969
<< <i>PS: thanks for not including the full-blown images. You might have shut down the CU forum bandwidth! >>
Full blown ones are 1.5 megs each
<< <i>The "O" is weak and the PLUR looks weak. Is that how it looks or is it just the pic??? >>
They are a little weak--does it make a difference that it was graded quite a few years ago (fatty slab)? Maybe the color gave a boost?
Jonesy, it very well may be a 66, but I've never seen another with toning like that, and I didn't pay 67 $$ for it, so I'm very happy with it. I'm trying to find it's D & S counterparts, to no avail thus far
I'm really wondering if that coin toned naturally or maybe even if the slab caused that type of toning. Who's the guy with, "Hey! watch out for those rattlers and no line NGC fatties" in his sig line? Maybe he knows something about this
Chiefbob, thanks for putting out the call to the chemistry experts as well, maybe one will swing in here and offer their opinion, as I would definitely appreciate it.
Thanks to all who posted!!
Rob
<< <i>PPS: can zinc tone? I thought it would only oxidize. Chemistry folks? >>
I have spoken with my father, a PhD. in organic chemisty...
Zinc can tarnish with sulfur in the air, making zinc sulfide, which is generally dark. However, if the layers (we're talking super-thin here) are arranged in certain ways, light can hit the layers, and certain colors can negate each other, leaving only another color. For example, if the reds negate, you might only see green in a certain spot where, if nothing cancelled, you'd see a dark mixture of green and red...
Thus, it is possible that these colors are simply caused by layers counteracting each other, leaving neon behind.
Jeremy
I think I understand
reflections and refractions, right?
Rob
<< <i>reflections and refractions, right?
I think so